Figures & data
Figure 1 Physical and covalent conjugation of thrombin and bFGF to the γ-Fe2O3 nanoparticles. ≈, ~, and – are symbols for precipitation, physical binding and covalent binding of various ligands to the γ-Fe2O3 nanoparticles, respectively.
Abbreviation: bFGF, basal fibroblast growth factor.
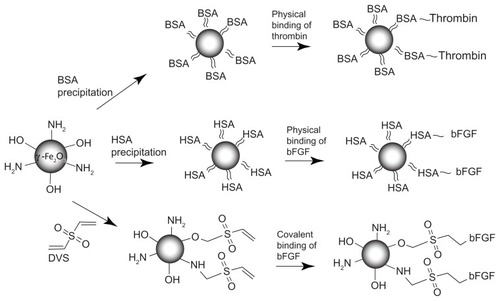
Figure 2 HRTEM images of the γ-Fe2O3 (A and B), γ-Fe2O3≈BSA (C) and γ-Fe2O3≈ BSA~Thrombin (D) nanoparticles placed on lacy grids. These images demonstrate the gelatin (A and B), gelatin≈BSA (C) and gelatin≈BSA~Thrombin (D) coatings on the crystalline γ-Fe2O3 core of the nanoparticles.
Abbreviations: HRTEM, high-resolution transmission electron microscopy; BSA, bovine serum albumin.
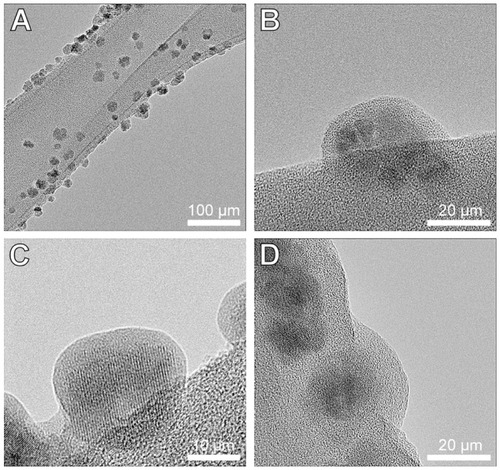
Table 1 The concentrations and the conjugation yields of the thrombin and the bFGF bound to the γ-Fe2O3 nanoparticles
Figure 3 Immunofluorescence double staining of NOM cells cultured for 21 days in 2D stationary culture, prior to their seeding in the magnetic fibrin scaffolds. (A1) S100B (glia marker in red); (A2) NF (neuronal marker in green); (A3) merge of S100B and NF. (B1) Nestin (neuronal precursor marker in red); (B2) β3Tub (neuronal marker in green); (B3) merge of nestin and β3Tub. The nuclei of the cells were dyed in blue with DAPI.
Abbreviations: NOM, nasal olfactory mucosa; 2D, two-dimensional; S100B, 100% soluble calcium-binding protein B; NF, neurofilament; β3Tub, beta III tubulin; DAPI, 4′,6-diamindo-2-phenylindole.
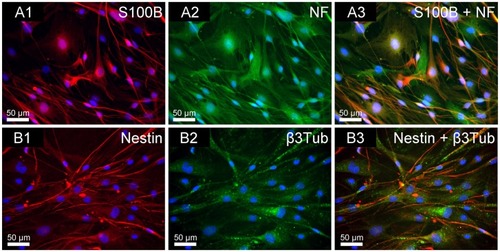
Figure 4 Quantitative analysis of the NOM cells adhered to the magnetic fibrin scaffold coating, or PLL coating, or uncoated culture plate (control) at different time intervals post-seeding. NOM cells were seeded in 24-well culture plates coated with the magnetic fibrin hydrogel or with PLL, or uncoated wells. 2, 4, 6, and 24 h after the seeding the wells were rinsed with the culture medium to remove the non-adherent cells. Quantification of the number of the adherent cells was performed by phase-contrast microscope images of five random non-overlapping fields of each well. Cells were then counted using ImageJ software, and the average and the standard deviation were calculated.
Abbreviations: NOM, nasal olfactory mucosa; PLL, poly-L-lysine.
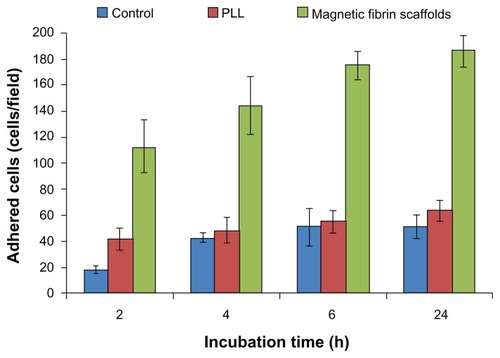
Figure 5 TEM (A) and SEM (B and C) images of the magnetic fibrin hydrogel scaffolds in the absence (A and B) or presence (C) of NOM cells. The magnetic fibrin scaffolds were prepared by the interaction of thrombin-conjugated γ-Fe2O3 nanoparticles with fibrinogen as described in the experimental part. The arrows in (A) point to the thrombin-conjugated nanoparticles.
Abbreviations: NOM, nasal olfactory mucosa; SEM, scanning electron microscopy; TEM, transmission electron microscopy.

Figure 6 Magnetization curves at room temperature of the magnetic fibrin hydrogel scaffolds containing 0.15% (A) and 1.5% (B) γ-Fe2O3 nanoparticles. (A) represents the magnetic fibrin hydrogel scaffolds containing the thrombin-conjugated γ-Fe2O3 nanoparticles only; (B) represents the magnetic fibrin hydrogel scaffolds containing in addition to the thrombin conjugated nanoparticles also the bFGF conjugated γ-Fe2O3 nanoparticles.
Abbreviation: bFGF, basal fibroblast growth factor.
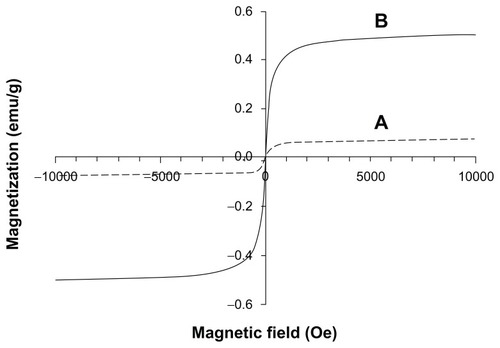
Figure 7 Attraction of the magnetic fibrin hydrogel scaffolds containing 0.15% (top pictures) or 1.5% (bottom pictures) of the γ-Fe2O3 nanoparticles to a magnet. The top pictures represent the magnetic fibrin hydrogel scaffolds containing the thrombin-conjugated γ-Fe2O3 nanoparticles only. The bottom pictures represent the magnetic fibrin hydrogel scaffolds containing in addition to the thrombin conjugated nanoparticles also the bFGF conjugated γ-Fe2O3 nanoparticles. The arrows point to the magnetic fibrin scaffolds.
Abbreviation: bFGF, basal fibroblast growth factor.
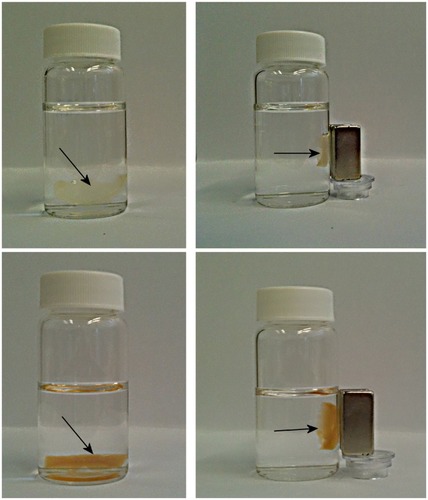
Figure 8 SEM images illustrating the NOM cells attached to the bFGF-γ-Fe2O3/MCs aggregates before being transferred to the magnetic fibrin scaffolds. (B) represents higher magnifications of the circulated area shown in (A).
Abbreviations: bFGF, basal fibroblast growth factor; MC, chitosan microcarriers; NOM, nasal olfactory mucosa; SEM, scanning electron microscopy.
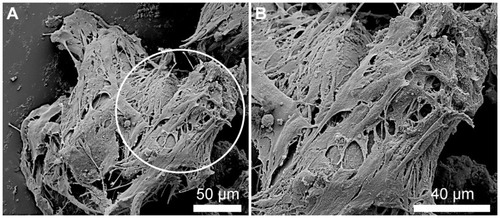
Figure 9 Phase-contrast microscope images illustrating the effect of the free and conjugated bFGF on the migration and growth of the NOM cells from the cells/free or conjugated bFGF/MCs aggregates, 18 days after the cultivation of the cell aggregates in the magnetic fibrin hydrogel scaffolds. (A) exhibits the effect of the naked γ-Fe2O3 nanoparticles (control), (B) the effect of the physically conjugated bFGF nanoparticles, (C) the effect of the covalently conjugated bFGF nanoparticles. The effect of the same concentration of the free factor as that of the conjugated bFGF, and 5 and 10 times higher is illustrated in images (D), (E), and (F), respectively.
Note: The asterisks indicate some of the NOM cells/bFGF-γ-Fe2O3/MCs aggregates.
Abbreviations: bFGF, basal fibroblast growth factor; MC, chitosan microcarriers; NOM, nasal olfactory mucosa.
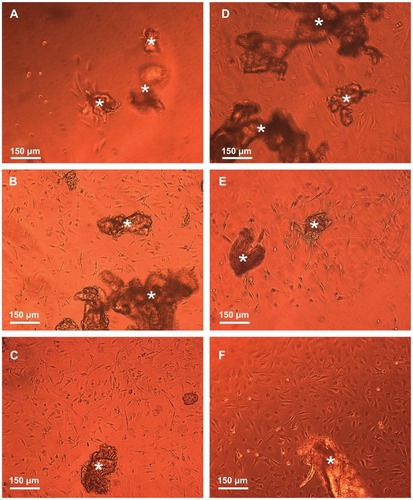
Figure 10 Quantitative analysis of the NOM cells migrated from the cells/nonconjugated or bFGF-conjugated nanoparticles/MCs aggregates and from the cells/different concentrations of the free factor/MCs aggregates (same concentration as the conjugated factor and 5 and 10 times higher), 5, 11, and 18 days after the cultivation of the cell aggregates in the magnetic fibrin hydrogel scaffolds. The reported values are an average of measurements performed on at least three randomly nonoverlapping fields of each triplicate tested culture.
Note: ~ and – are symbols for physical and covalent bindings, respectively, of the bFGF to the γ-Fe2O3 nanoparticles.
Abbreviations: bFGF, basal fibroblast growth factor; MC, chitosan microcarriers; NOM, nasal olfactory mucosa.
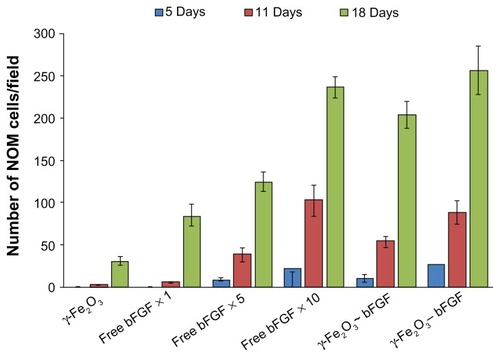
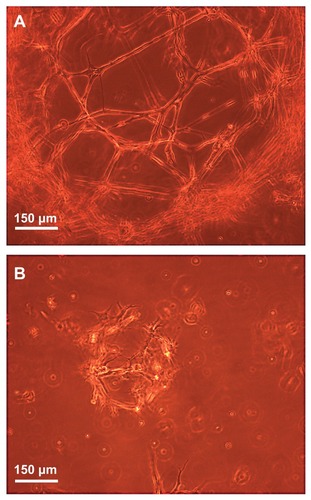
Figure 11 Phase-contrast microscope images illustrating the three-dimensional growth, proliferation, and differentiation of the NOM cells in the magnetic fibrin hydrogel scaffolds 21 days postcultivation in the presence (A) and absence (B) of the bFGF-γ-Fe2O3 nanoparticles.
Abbreviations: bFGF, basal fibroblast growth factor; NOM, nasal olfactory mucosa.