Figures & data
Figure 1 Schematic presentation of the chemical reactions during the preparation of poly(propylene adipate)-block-poly(ɛ-caprolactone) copolymers.
Abbreviation: PPAd, poly(propylene adipate).
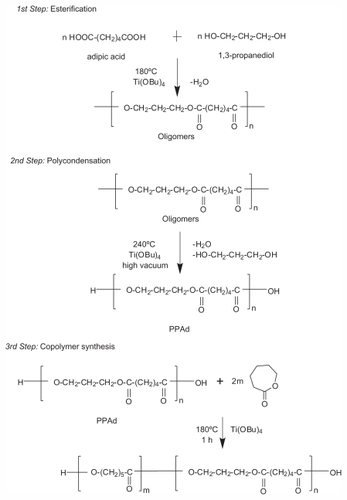
Table 1 Theoretical and calculated compositions, intrinsic viscosities, and molecular weights of prepared polymers
Figure 2 Hydrogen-1 nuclear magnetic resonance (1H NMR) spectra of poly–(ɛ-caprolactone) (PCL), poly(propylene adipate) (PPAd), and PPAd/PCL 85/15 weight/weight copolymer (A) and 1H NMR spectra of PPAd/PCL 85/15 weight/weight copolymer at the region 3.98–4.16 ppm (B).
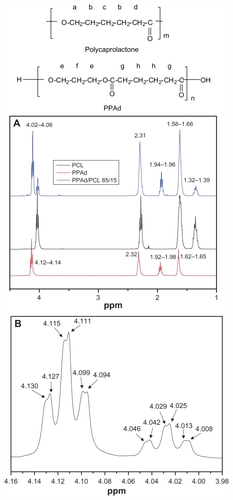
Figure 3 Carbon-13 nuclear magnetic resonance (13C NMR) spectrum of poly(ɛ-caprolactone) (PCL), poly(propylene adipate) (PPAd), and their copolymer PPAd/PCL 65/35 weight/weight (A) and 13C-NMR spectrum at region 173 ppm of PPAd/PCL 65/35 weight/weight (B).
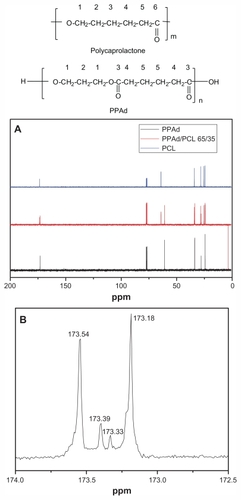
Table 2 Composition, probabilities of triads, randomness factor, and the average number sequence lengths of synthesized copolyesters
Table 3 Glass transition temperature, melting temperature, heat of fusion values, and degree of crystallinity of poly(ɛ-caprolactone), poly(propylene adipate), and their copolymers
Figure 4 X-ray diffraction patterns for synthesized polyesters.
Abbreviations: PCL, poly(ɛ-caprolactone); PPAd, poly(propylene adipate).
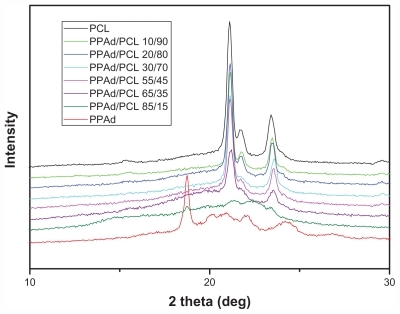
Figure 5 Differential scanning calorimetric thermograms of neat polymers and synthesized copolymers.
Abbreviations: PCL, poly(ɛ-caprolactone); PPAd, poly(propylene adipate).
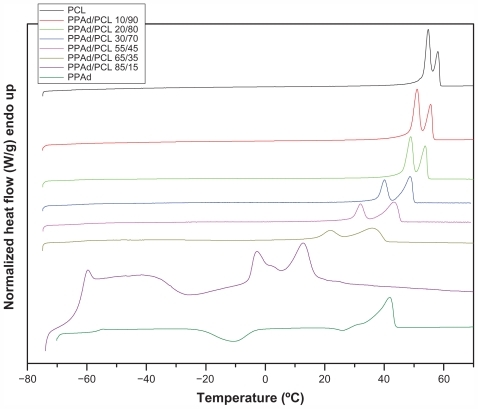
Figure 6 Viability of human umbilical vein endothelial cells as a function of copolymer type and concentration (cell incubation time: 24 hours).
Abbreviations: PCL, poly(ɛ-caprolactone); PPAd, poly(propylene adipate).
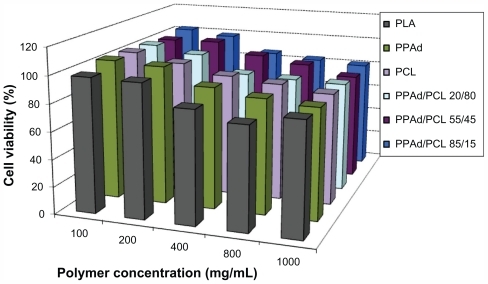
Table 4 Characteristics of prepared desferrioxamine-loaded nanoparticles such as average diameter, polydispersity index, zeta potential, nanoparticles yield (%), drug loading (%), and entrapment efficiency (%)
Figure 7 Scanning electron microscope photos from desferrioxamine-loaded poly(ɛ-caprolactone) (A) and poly(propylene adipate)/poly(ɛ-caprolactone) 65/35 weight/weight (B) nanoparticles.
Abbreviation: SEI, secondary electron imaging.
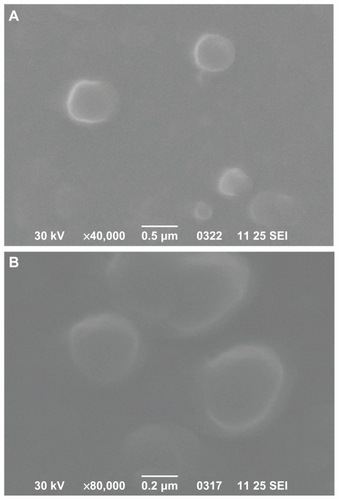
Figure 8 High-resolution transmission electron microscope photos from prepared nanoparticles poly(propylene adipate)/poly(ɛ-caprolactone) 30/70 weight/weight.
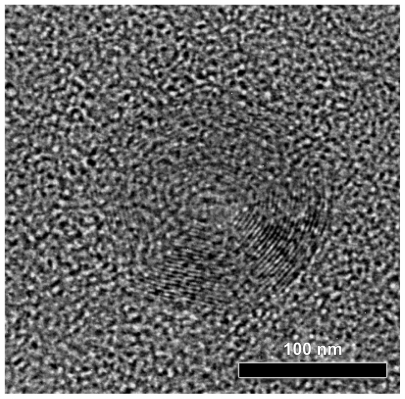
Figure 9 X-ray diffraction patterns of desferrioxamine mesylate and drug-loaded nanoparticles.
Abbreviations: PCL, poly(ɛ-caprolactone); PPAd, poly(propylene adipate).
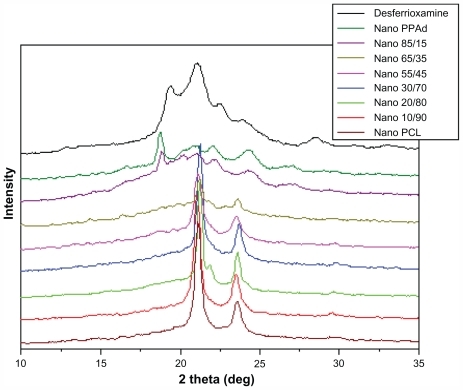
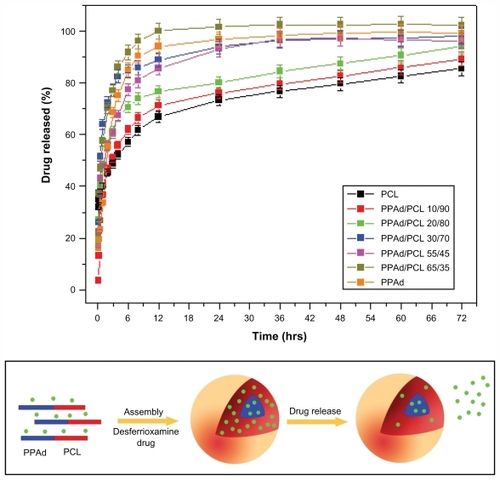
Figure 10 (A) Dissolution profiles of desferrioxamine from prepared nanoparticles. The release rate in copolymer containing 85 weight percent poly(propylene adipate) (PPAd) was not possible to study due to the softness of the material. (B) Illustration of possible distribution patterns of drug in the self-assembly PPAd/poly(ɛ-caprolactone) (PCL) nanoparticles based high-resolution transmission electron microscope micrographs and its possible influence on early drug release from the nanoparticles.