Figures & data
Table 1 Composition of Different Niosomal Formulations
Figure 2 DSC thermograms of Span 20, Brij 35, physical mixture 3 (PM3), physical mixture 4 (PM4), F7, F8, F9, F10 and F11 formulations.
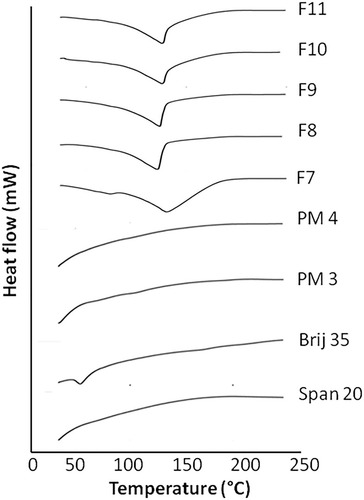
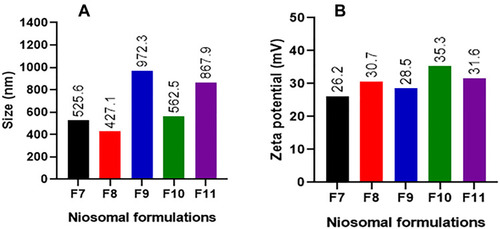
Table 2 Mean Size, Polydispersity Index and Zeta Potential of Niosomal Formulations
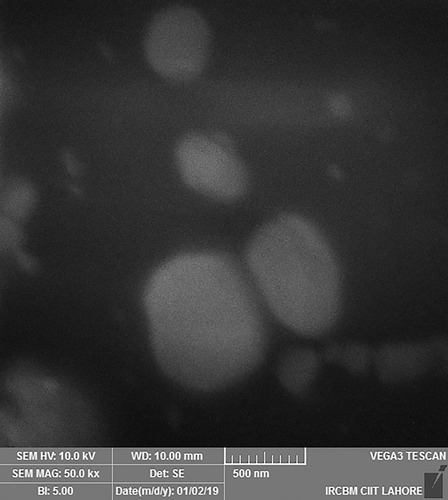
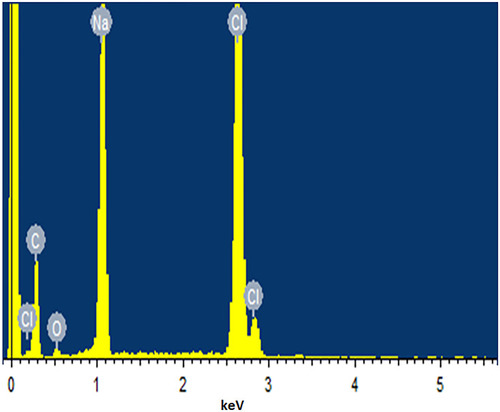
Table 3 Atomic % and Weight % of Elements in EDX Spectra of Formulation F10
Table 4 Mean Entrapment Efficiency of Niosomal Formulations
Table 5 Change in Vesicle Size, PDI and Zeta Potential of Niosomal Formulations F7-F11 at Temperature 4–8 °C in Three Months
Table 6 Change in Size, PDI and Zeta Potential of Preparations F7-F11 at Temperature 25± 2 °C in Three Months
Table 7 Stability Studies of Cyclosporine a Niosomes at Different Temperatures
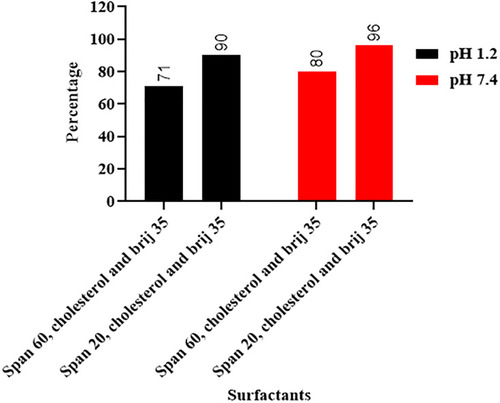
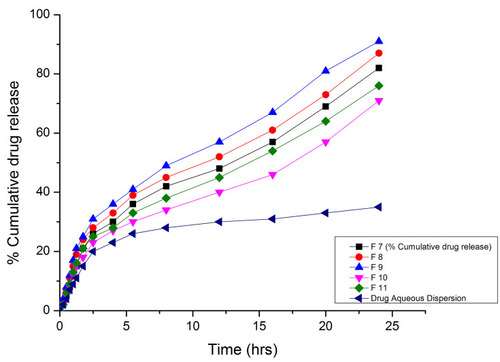
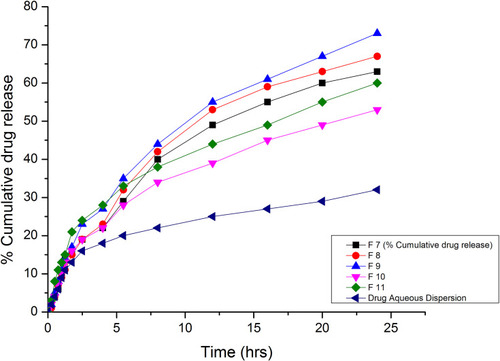
Table 8 Drug Release Data and Kinetic Modeling of Formulations F7-F11 at pH 1.2 and 7.4
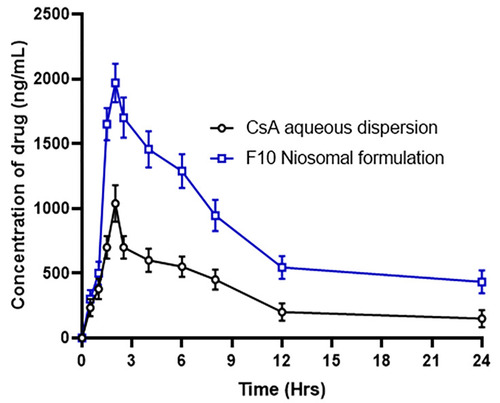
Table 9 Mean Pharmacokinetic Parameters of Niosomal Formulations F10 and CsA Aqueous Dispersion Administered in Dose (10 Mg/Kg) Orally to Albino Rabbits (n=6)