Figures & data
Figure 1 The HPV genome and gene functions. HPVs are typical non-enveloped double-stranded DNA viruses, with circular and approximately 8000 pairs in size. Most encode eight major genes, six genes located in the early regions and two in the late regions. The early genes can regulate the HPV genome replication and transcription, viral release, celling signaling and viral apoptosis, immune modulation, and structural modification of infected cells.Citation60
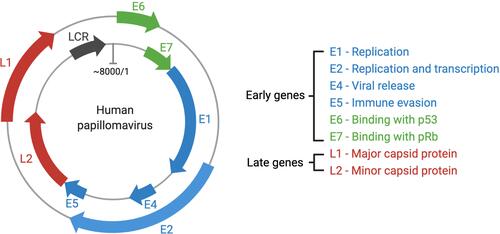
Figure 2 (A) Structure of HPV; (B) changes of epithelial cells in the cervix following HPV infection. (B): a. Viral infection and its early gene expression phase: HPV virus invade the basal layer of the stratified epithelium and initiates early gene (E1 and E2) expression. The oncogenic virus quickly amplifies into 50–100 copies per cell in E1- and E2-dependent manners, with a low copy number of HPV episomes maintained via replication with cellular DNA; b. Viral gene latter expression and amplification phase: one infected cell remains in the basal layer, while other cells continue to enter the suprabasal layer. The episomal DNA sequence of HPV diffuses into the nucleus of infected cells. There, it undergoes genetic replication and assembly; c. Viral particle assembly and release phase: the life cycle is directly controlled by differentiation of the host cell, where HPV viruses are released from keratinocytes.
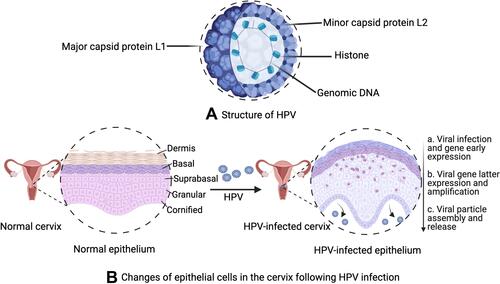
Figure 3 Human immune responses against invasive pathogens. The human immune system has been classified into two general groups, including innate and adaptive immune responses. Innate immune cells stimulate rapid reactions, whereas adaptive immune cells have a delayed response, producing immunological memory.Citation86 Rapid responding innate cells include polymorphonuclear cells, mast cells, macrophages, and dendritic cells, which are capable of internalizing and destroying invading microbes as well as the secretion of cytokines and proinflammatory chemokines, inducing other immune cells to the site of infection. Macrophages and DCs, as antigen-presenting cells (APCs), is capacity to ingest pathogens and produce pathogen peptides on their cell surface, which can be recognized by major histocompatibility complex (MHC) class I and MHC-II, with the induction of cellular immune response and humoral immune response, respectively.
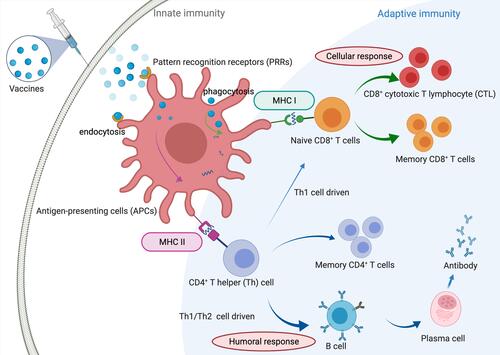
Table 1 Features of the Most Common Therapeutic Vaccine Candidates Against Cervical CancersCitation6,Citation99,Citation100
Figure 4 Schematic process of the development of peptide-based vaccines. The whole and successful process of developing peptide-based vaccines is classified into six main steps, including the identification of pathogens and target proteins, selection of antigen epitopes, vaccine formulation to build vaccine candidates, in vivo trials of peptide-based vaccine candidates, clinical trials of vaccine candidates, and final vaccination and immunization in humans against infectious diseases.
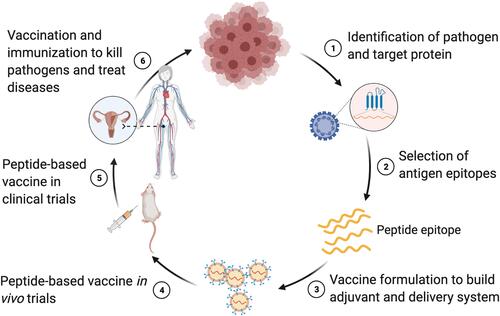
Table 2 Epitope Sequences of Peptide-Based Therapeutic HPV Vaccines
Table 3 The Use of Immunological Adjuvants in Peptide-Based Nanovaccines Against Cervical Cancer
Figure 5 Different peptide-based nanostructures, loading strategies and mechanism. Peptide-based nanostructures include lipid-based nanoparticle, dendrimer, polymeric micelle, nanosphere, polymeric nanoparticle and virus like particle. Peptide-based loading strategies include physical mixture, encapsulation, adsorption, self-assembly and chemical conjugation. Mechanisms of peptide-based nanovaccines in the treatment of cervical cancer include surface pattern recognition receptors (PRRs) activation, endosomal TLRs activation, inflammasome activation, immune cell recruitment and enhance antigen uptake.
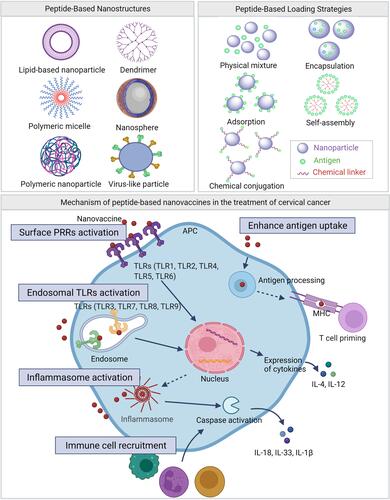
Table 4 Clinical Trials of Peptide-Based Vaccines Against Cervical Cancer
