Figures & data
Figure 1 Characterization of targeting micelles. (A) The chemical structure of the telodendrimer. Dendritic oligomers of cholic acid are conjugated to the right ends of polyethylene glycol (PEG) while PLZ4 is at the left. The facial amphiphilic structure of cholic acid with the hydrophilic hydroxyl groups (red) at one side and hydrophobic methyl groups (blue) at the other side allows the formation of spheric micelles with PLZ4 displayed on the surface. (B) The spherical micelle structure with PLZ4 on the surface for targeting and the central space for loading of imaging and/or therapeutic agents. (C) Dynamic light scattering showing the size of targeting micelles (23.2 ± 8.1 nm) with narrow distribution. (D) Transmission electron microscopy imaging.
Note: Bar length is 50 nm.
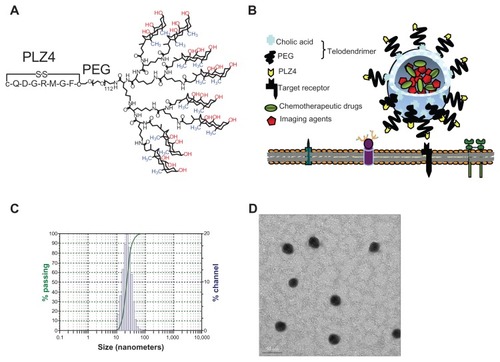
Figure 2 More efficient delivery of targeting micelles into bladder cancer cell lines. (A) K9TCC-Pu-In, (B) K9TCC, and (C) K9TCC-Pu-Axc cells were incubated with various concentrations of nontargeting (NM) or targeting (PLZ4-NM) micelles, both loaded with DiD, for 1 hour before washing and analysis using an enzyme-linked immunosorbent assay reader.
Note: Targeting micelles were more efficient in drug delivery than nontargeting micelles in a dose-dependent pattern (*P < 0.05).
Abbreviation: DiD, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine.
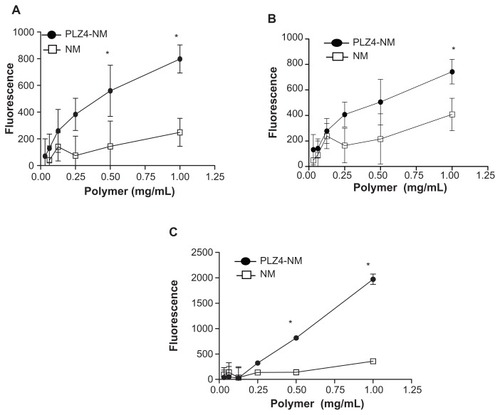
Figure 3 Cell uptake of nontargeting and targeting micelles by dog bladder cancer cells. K9TCC-Pu-In cells were incubated with nontargeting (NM-DiD) or targeting micelles (PLZ4-NM-DiD), both loaded with DiD/PTX, for 1 hour before washing and imaging. (A) Comparison of cell uptake between nontargeting and targeting micelles with K9TCC-Pu-In (left) and normal dog primary urothelial cells (right) showing little retention of micelles with normal urothelial cells. The experiments were repeated three times with the cell line and twice with primary urothelial cells (100×). (B) High-resolution tomography showed the difference in the cell uptake of targeting (PLZ4-NM-DiD, upper panel) and nontargeting (NM-DiD, lower panel) micelles in K9TCC-Pu-Axc cells. 4′,6-diamidino-2-phenylindole (blue, nuclear staining) and white light phase images were presented to show nucleus and cell morphology, respectively.
Notes: The white arrow points to the attachment of targeting micelles to the cell membrane. Bar = 15 μm.
Abbreviations: DiD, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine; PTX, paclitaxel.
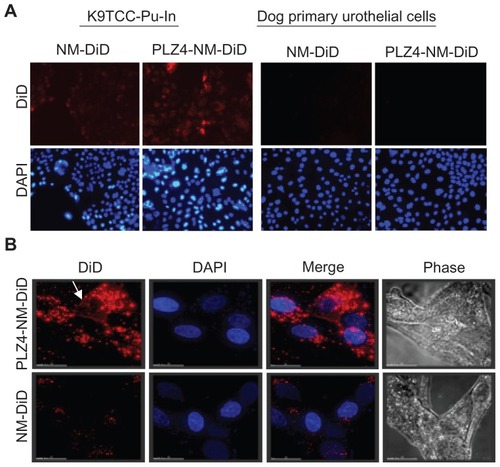
Figure 4 Cell cytotoxicity assay of drug-loaded micelles on dog bladder cancer. K9TCC-Pu-In cells were seeded and cultured overnight before being treated with various concentrations of nontargeting or targeting micelles loaded with daunorubicin (DNR) for two hours at 37°C. Cells treated with empty micelles and free DNR under the same conditions served as controls. Cells were then washed and cultured with complete culture medium for 72 hours and the cell viability was evaluated using the WST-8 assay according to the manufacturer’s protocol. The absorbance was detected using an enzyme-linked immunosorbent assay reader. This experiment was performed in triplicate and repeated three times.
Notes: *P = 0.02 for free DNR versus PLZ4 micelles and 0.04 for free DNR versus nontargeting micelles. **P = 0.001 for free DNR versus PLZ4 micelles; 0.01 for free DNR versus nontargeting micelles; and 0.04 for nontargeting versus targeting micelles.
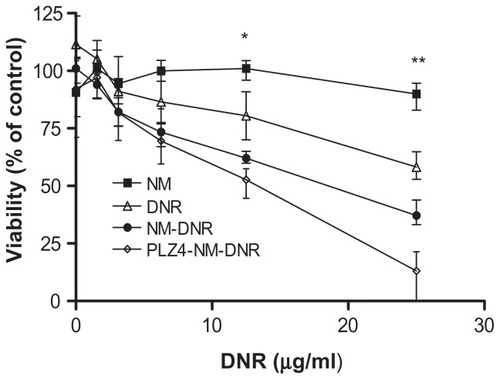
Figure 5 Establishment of dog orthotopic bladder cancer xenograft model in mice. Whole bladders were harvested on days 1 (A and D), 7 (B and E), and 20 (C and F) after injection of cancer cells for hematoxylin and eosin staining and examination. Blue arrows show an intact normal urothelial layer lining the bladder cavity on day 1 and replacement of the normal urothelial layer and bladder cavity by xenograft on day 20. Blue stars show the injection (day 1) and expansion of cancer cells (day 7) at the lamina propria and invasion into the muscle layer on day 20. (A, B and C: 4×; D, E, and F: 40×). (G) urine sediment cytology evaluation on day 20 (40×). (H) The touch preparation smear from the solid tumor in the bladder at day 20 (40×).
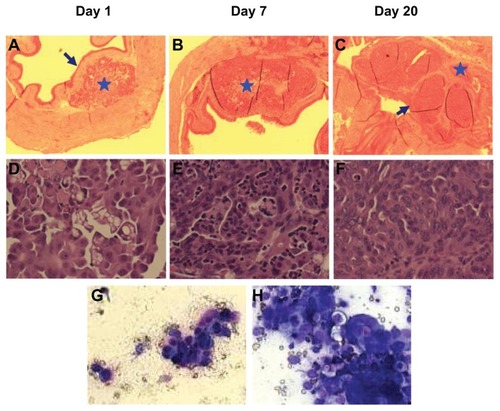
Figure 6 In vivo and ex vivo imaging on an orthotopic dog invasive bladder cancer model in mice. (A) In vivo imaging of mice 24 hours after receiving free DiD dye or nontargeting (NM-DiD) or targeting (PLZ4-NM-DiD) micelles loaded with PTX/DiD. Mice were covered to protect bladder xenografts and to prevent evaporation of vital organs. (B) Ex vivo imaging of the tumor/bladder and other major organs 24 hours after injection. The color bar represents the relative fluorescence strength. (C) The average fluorescence strength of whole tumor/bladder for free DiD, NM-DiD, and PLZ4-NM-DiD was calculated from three different mice (*P < 0.05). (D) To eliminate other variations, the fluorescence signals of xenografts were normalized with the signals of the liver, lungs, and muscles of the same mice (*P < 0.05).
Abbreviations: DiD, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicar bocyanine; PTX, paclitaxel.
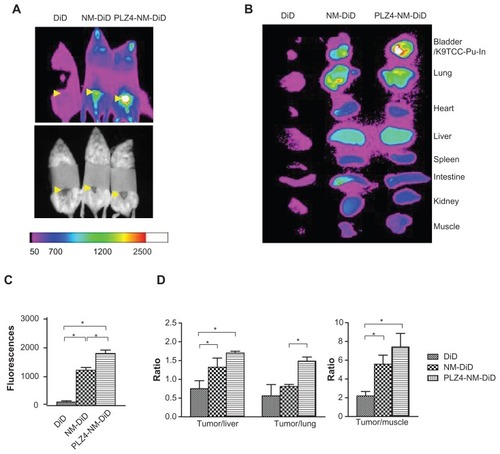
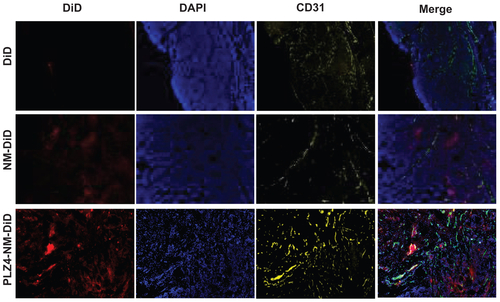
Figure S1. Intratumoral distribution of micelles. After ex vivo imaging of tumor xenografts, cryosections (10 μm thick) were obtained and the samples were fixed with 10% formalin for 10 minutes at room temperature. After 30 minutes of blocking in 1% BSA/PBS, samples were incubated with 1:100 antiCD31 (blood vessel endothelium cell marker) antibody (Millipore, Billerica, MA) for 1 hour at room temperature. Goat antimouse IgG conjugated Cy3 was used as a secondary antibody for another 1 hour at room temperature. Before observing using the microscope, slides were mounted using DAPI-containing antifading mounting medium. The imaging was acquired using Metamorph Microscopy Automation and Imaging analysis software (Molecular Devices). The distribution of DiD/micelles is shown as red, while the vascular endothelial marker CD31 is shown as yellow and the nucleus/DAPI as blue in the xenografts from mice treated with free DiD (upper panel), nontargeting micelles (NM-DiD, middle panel), and PLZ4-targeting micelles (PLZ4-NM-DiD, lower panel). The very right panel shows the merged imaging.
Abbreviations: BSA/PBS, bovine serum albumin/phosphate-buffered solution; DAPI, 4′,6-diamidino-2-phenylindole; DiD, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicar bocyanine.