Figures & data
Table 1 Composition of the 33 CCD for DLX in situ Cubo-Gels
Table 2 Average PS, GT, EE%, Q6, PDI and ZP for the Prepared DLX in situ Cubo-Gels
Figure 1 3D surface plot for the main effects and interactions of lipid ratio, PF127 percentage, and PF68 percentage on (A) PS (B) GT, (C and D) EE%, and (E) Q6. The lipid ratio had a significant effect on PS, EE%, and Q6; the PF127% had a significant impact on PS, GT, and EE%; the PF68% had a significant effect on EE% and Q6.
Figure 2 In vitro release profile of DLX in situ cubo-gels in PBS (pH = 7.4) at 37°C. Q6 ranged from 20.3 ± 2.14 to 64.5 ± 4.13% and the release extent ranged from 42.77 ± 7.01 to 101.38 ± 1.7%.
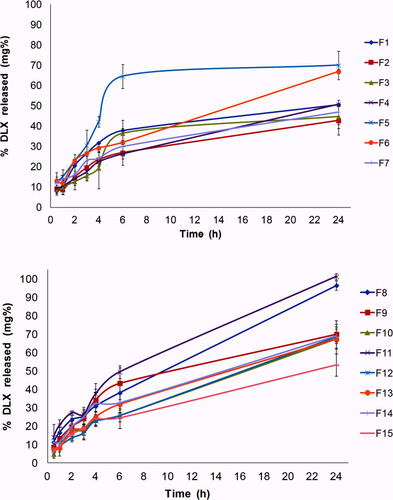
Figure 3 TEM images of (A) optimized DLX in situ cubo-gel formulation and (B) magnified single cubosome. The images show the cubosomes with numerous water channels in their structure.
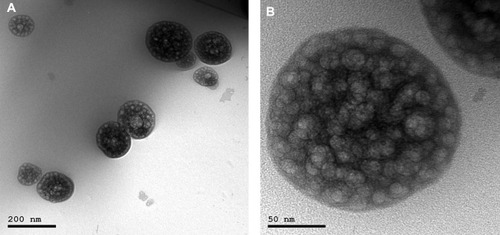
Figure 4 DSC thermograms of DLX showing an endothermic peak at 167.87°C and of optimum DLX in situ cubo-gel showing the disappearance of the DLX endothermic peak.
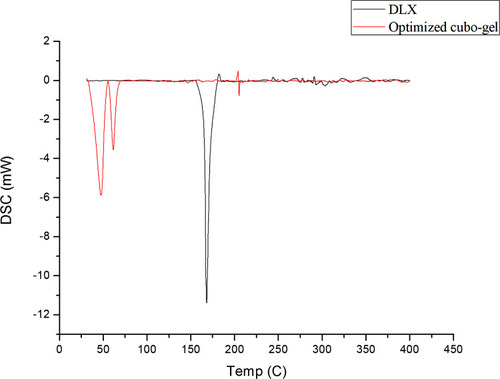
Figure 5 FTIR spectra of DLX and optimum DLX in situ cubo-gel showing shifted and reduced peaks at 1577.77 (aromatic alkenes), 1462.04 (thiophene ring), and 1234.44 (carbonyl group).
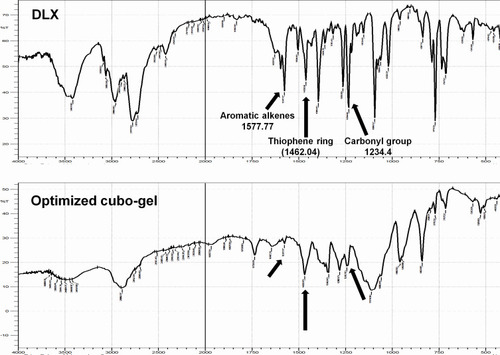
Figure 6 XRPD of DLX showing distinct peaks at 18.2°, 19.07°, 21.08°, 23.5°, and 28.1° and of optimum DLX in situ cubo-gel showing the disappearance of these peaks.
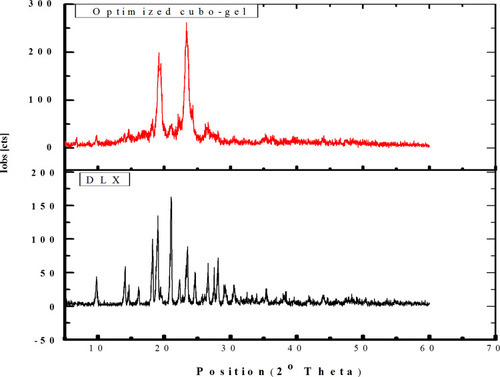
Figure 7 Cell viability of the optimized DLX in situ cubo-gel compared to plain in situ cubo-gel and DLX solution on oral epithelial cells showing that the plain in situ cubo-gel had a significantly higher IC50 (70.85 μg/mL) compared to the DLX solution and the DLX in situ cubo-gel, which had almost similar IC50 values (21.66 and 20.77 μg/mL, respectively).
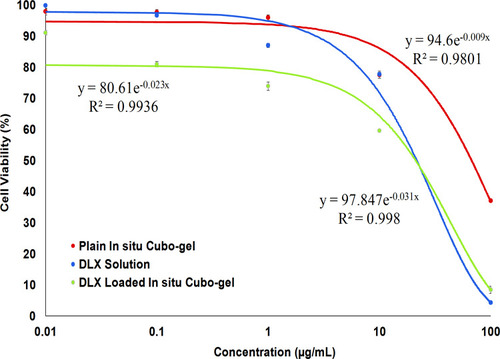
Figure 8 Cumulative amount of DLX permeated per unit area across the nasal sheep membrane via the optimized DLX in situ cubo-gel compared to the DLX solution showing increased permeation with 1.27 enhancement ratio.
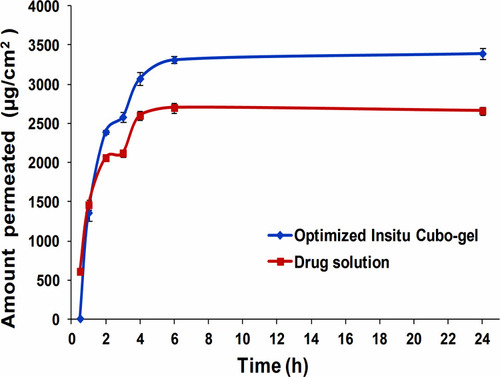
Table 3 Pharmacokinetic Parameters of DLX in Plasma and Brain Homogenate After Intranasal and Intravenous Administration of the Optimized in situ Cubo-Gel and Drug Solution
Figure 9 Mean plasma concentration-time profiles of IN DLX in situ cubo-gel in comparison to IN solution, IV formula, and IV solution after the administration in Swiss albino rats. The in situ cubo-gel showed higher AUC0-inf compared to the IN solution with relative bioavailability of 188.92%.
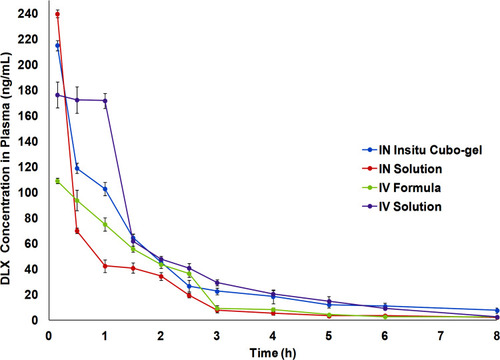
Figure 10 Mean brain homogenate concentration-time profiles of IN DLX in situ cubo-gel in comparison to IN solution, IV formula, and IV solution after the administration in Swiss albino rats. The in situ cubo-gel showed higher AUC0-inf compared to the IN solution with relative bioavailability of 196.13%.
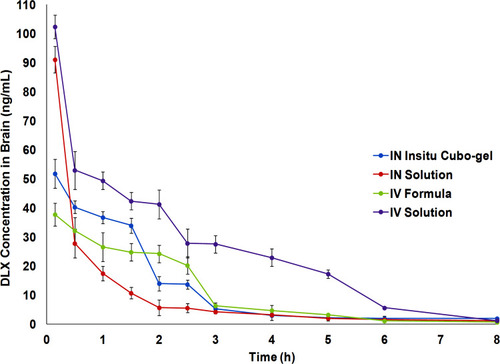
Table 4 Average PS, ZP, PDI and EE% of Optimum DLX in situ Cubo-Gel After Storage for 3 Months at Room Temperature and Refrigerator
