Figures & data
Table 1 Main Types and Mechanisms of Drugs Used for Bacterial Infections
Figure 1 Types of oral antibiotic nanopreparations and mechanisms by which oral absorption improvement of antibiotics through nano-drug delivery system.
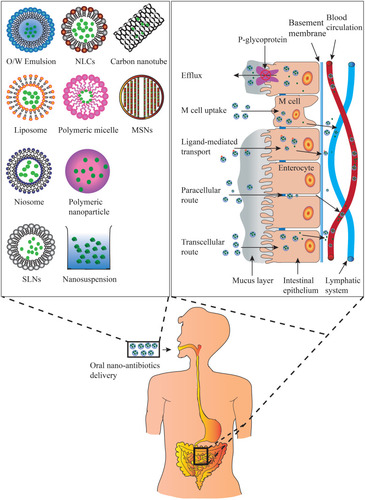
Table 2 Composition and Properties of Lipid-Based Nanocarriers for Oral Delivery of Antibiotics
Figure 2 Delivering amoxicillin at the infection site – a rational design through lipid nanoparticles. (A) AMX-loaded LNPs, which were designed to release AMX near H. pylori. The double-emulsion LNPs are composed of cetyl palmitate, Tween 80, linolenic acid, and DOPE. Abbreviations: AMX, amoxicillin; DOPE, dioleoylphosphatidylethanolamine; H. pylori, Helicobacter pylori; LNPs, lipid nanoparticles. (B) In vitro AMX release profiles from the LNPs in three simulated conditions, namely 1) pH 1.6, 2) pH 5.0, and 3) pH 7.4. Notes: Vertical lines represent media changes. Values represent the mean ± SD of three independently produced formulations. (C) In vitro cell viability studies. L929 cell viability study and MKN-74 cell viability study. Different formulations in different solid lipid concentrations, from 0 (black) to 8 (light gray) mg/mL of solid lipid were evaluated. For free AMX, the same amount of AMX existent in those concentrations of LNPs was used, with the exception of 1 and 4 mg/mL. Notes: Values represent mean ± SD of three independently produced formulations. *P<0.05, **P<0.01, ***P<0.005, ****P<0.0001 relative to 0 mg/mL. Notes: Vertical lines represent media changes. Values represent the mean ± SD of three independently produced formulations. (D) Characterization of the AMX-loaded LNPs suspensions before (dark gray) and after (light gray) the incubation with mucins. 1) LNPs size and PDI. Bars represent the size (left y-axis) and dots represent the PDI (right y-axis). 2) LNPs zeta potential. Notes: Values represent the mean ± SD of three independently produced formulations. *P<0.05, **P<0.01, ****P<0.0001 relative to the LNPs without mucins. Abbreviations: PDI, polydispersity. (E) TEM images of the AMX-loaded LNPs and the corresponding unloaded LNPs. The difference among the four formulations (F1–F4) was their composition relative to the presence or absence of linolenic acid and DOPE. P1–P4 stands for AMX unloaded LNPs. P1, F1, P2, F2, F3, P4A, and F4A are at a magnification of 50,000×. P3 is at a magnification of 25,000×. P4B and F4B are at a magnification of 100,000×. Copyright © 2019. Dove Medical Press. Reproduced from Lopes-de-Campos D, Pinto RM, Lima SAC, et al. Delivering amoxicillin at the infection site - a rational design through lipid nanoparticles. Int J Nanomedicine. 2019;14:2781–2795.Citation83
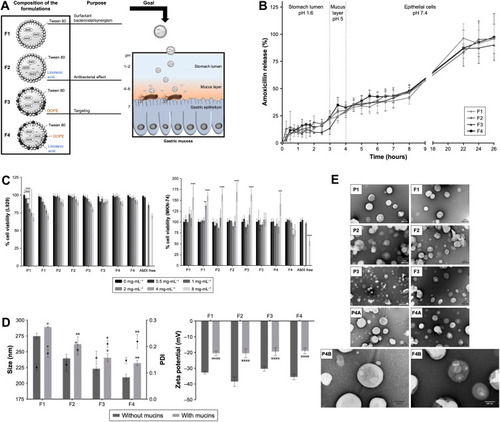
Figure 3 Solid lipid nanoparticles with enteric coating for improving stability, palatability, and oral bioavailability of enrofloxacin. (A) The production process of enrofloxacin enteric granules containing SLNs inner core. (B) Scanning electron microscopy photographs of enrofloxacin-loaded SLNs. (C) The accumulation release profiles of SLNs and granules in the simulated SIF (pH=8) (n=3). (D) The plasma enrofloxacin concentration profiles–time of the prepared granules and reference formulation (soluble powder) in pigs (n=6). (E) The influence of high temperature to release ability of enteric granules. (F) The influence of high humidity to release ability of enteric granules. (G) The influence of high light to release ability of enteric granules. SLNs, solid lipid nanoparticles; ENR, enrofloxacin; ENR-SLNs, enrofloxacin-loaded SLNs; RT, room temperature; PR, polyacrylic resin; SIF, simulated intestine fluid; Granules: 10% enrofloxacin enteric granules; Powder: 5% enrofloxacin-soluble powder; HT: high temperature (40°c); HI: high humidity (25°c, 90%±5%); HL: high light (4500±500 lx). Copyright© 2019. Li C, Zhou K, Chen D, et al. <p>Solid lipid nanoparticles with enteric coating for improving stability, palatability, and oral bioavailability of enrofloxacin. Int J Nanomedicine. 2019;14:1619–1631.Citation38
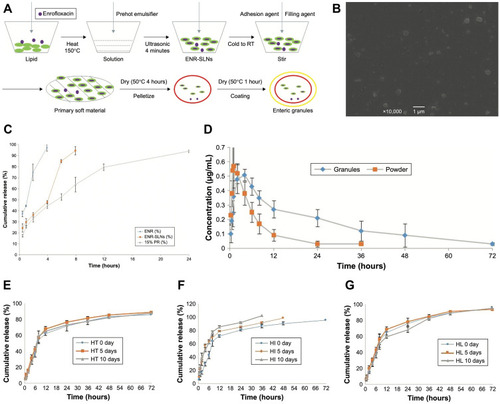
Table 3 Composition and Properties of Polymer-Based Nanocarriers for Oral Delivery of Antibiotics
Table 4 The Advantages and Disadvantages of Various Nanoparticle Delivery Systems
