Figures & data
Figure 1 (A) Ultraviolet and visible spectroscopy absorption spectrum of the chitosan-stabilized silver nanoparticles (CS-AgNPs) (maximum absorbance at 420 nm); (B) transmission electron microscopy image of CS-AgNPs (scale bar, 100 nm); (C) size distribution and (D) zeta potential of chitosan and CS-AgNPs determined by dynamic light scattering.
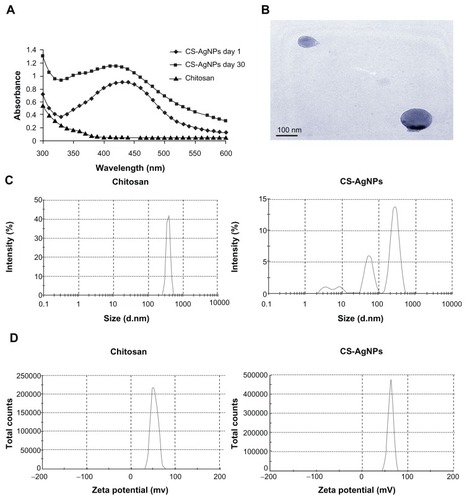
Figure 2 Dose-dependent killing of bacterial strains by chitosan-stabilized silver nanoparticles (CS-AgNPs): (A) Pseudomonas aeruginosa, (B) Salmonella typhi, (C) Staphylococcus aureus, and (D) Mycobacterium smegmatis were incubated with different concentrations of CS-AgNPs. Bacterial survival was determined at 1 and 4 hours by colony-forming unit (CFU) assay. Media containing bacteria alone (control) and chitosan plus bacteria (chitosan) were used as controls. (E) Antibacterial activity of CS-AgNPs by diffusion method: CS-AgNPs at 100 ppm (20 μL) were loaded into wells formed on plates containing a lawn of P. aeruginosa; growth inhibition was determined by measuring the zone of inhibition after 24 hours; chitosan was used as a control.
Notes: Experiments were performed in triplicate; results are shown as mean plus or minus standard deviation; *P ≤ 0.05.
Abbreviation: ND, bacterial growth not detected.
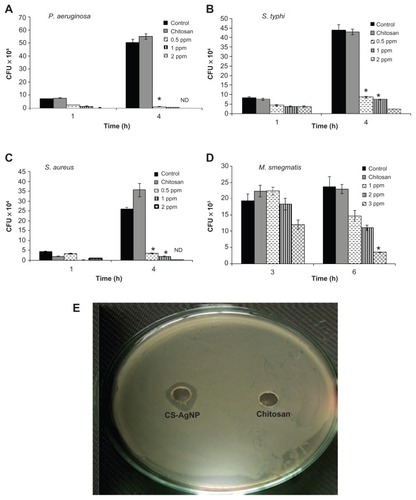
Figure 3 Scanning electron microscopy images of Pseudomonas aeruginosa after incubation with medium (control) and chitosan-stabilized silver nanoparticles (CS-AgNPs) (2 ppm) for 4 hours.
Note: Scanning electron microscopy analysis was performed on a SU1510 scanning electron microscope (Hitachi, Tokyo, Japan).
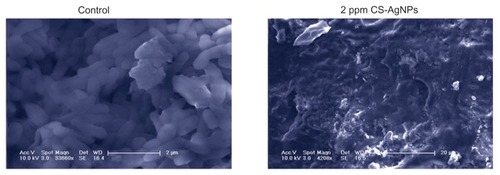
Figure 4 Effect of chitosan-stabilized silver nanoparticles (CS-AgNPs) on biofilm formation: the antibiofilm activity of CS-AgNPs was checked by incubating (A) Pseudomonas aeruginosa and (B) Staphylococcus aureus with different concentrations of CS-AgNPs for 24 hours in a 96-well plate. The addition of increasing concentrations of CS-AgNPs reduced the ability of the organisms to form biofilms.
Notes: Experiments were performed in triplicate; results are shown as mean plus or minus standard deviation.
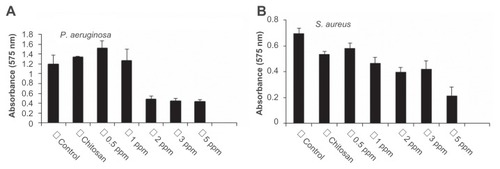
Figure 5 Cytotoxic activity of chitosan-stabilized silver nanoparticles (CS-AgNPs) on mouse macrophage RAW264.7 cells. Macrophages were treated with different concentrations of CS-AgNPs for 24 hours; cell viability was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay.
Notes: Experiments were performed in triplicate; results are shown as mean plus or minus standard deviation.
![Figure 5 Cytotoxic activity of chitosan-stabilized silver nanoparticles (CS-AgNPs) on mouse macrophage RAW264.7 cells. Macrophages were treated with different concentrations of CS-AgNPs for 24 hours; cell viability was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay.Notes: Experiments were performed in triplicate; results are shown as mean plus or minus standard deviation.](/cms/asset/f15802c7-2ff0-4384-93b3-298c9863d3bb/dijn_a_28077_f0005_b.jpg)
Figure 6 Genotoxic activity of chitosan-stabilized silver nanoparticles (CS-AgNPs) on mouse macrophage RAW264.7 cells: (A) DNA fragmentation of macrophages treated with different concentrations of CS-AgNPs for 6 hours – DNA was isolated from untreated (lane 1), chitosan (lane 2), and CS-AgNP-treated (3 and 10 ppm, lanes 3 and 4, respectively) macrophages and electrophoresed on agarose gel; (B) comet analysis – control and CS-AgNP-treated (3 and 20 ppm) macrophages stained with propidium iodide (4 μg/mL); (C) DAPI (4, 6-diamidino-2-phenylindole) staining of control and CS-AgNP-treated (3 and 10 ppm) macrophages.
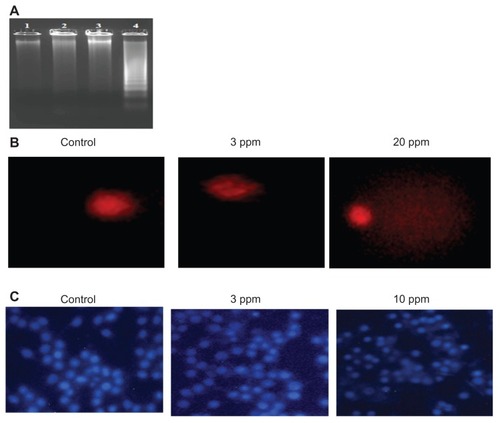
Figure 7 Chitosan-stabilized silver nanoparticles (CS-AgNPs) exhibit intracellular killing activity against Mycobacterium smegmatis. (A) Cellular uptake of propidium (PI)- labeled CS-AgNPs; (B) RAW264.7 macrophages were incubated with different concentrations of CS-AgNPs for 1 hour before (pretreatment) and after (post-treatment) M. smegmatis infection. Macrophages infected with bacteria alone were used as a control. The cells were lysed 6 hours post infection and bacterial intracellular survival was determined by colony-forming unit (CFU) assay.
Notes: Experiments were performed in triplicate; results are shown as mean plus or minus standard deviation; *P < 0.05.
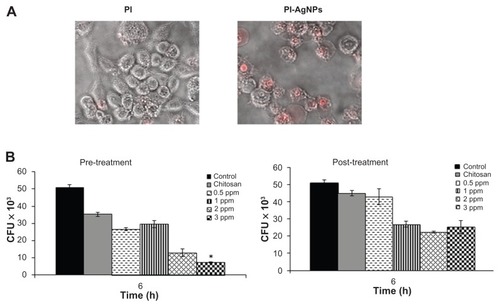
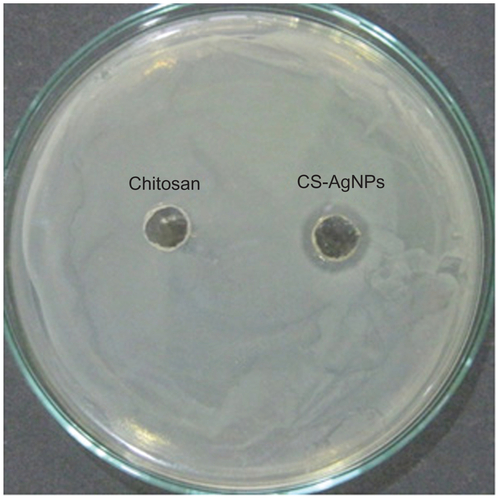
Figure S1 Antibacterial activity of silver nanoparticles (AgNPs) by agar diffusion method: chitosan-stabilized AgNPs (CS-AgNPs) at 2 ppm were loaded into the wells formed on plates containing a lawn of Staphylococcus aureus; growth inhibition was determined by measuring the zone of inhibition after 24 hours; chitosan was used as a control.