Figures & data
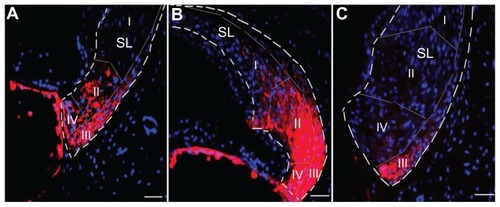
Figure 1 Illustration of polymersome schema and administration approaches. (A) Shows PEG-b-PCL block copolymers self-assembled in an aqueous environment to form polymersomes (PMs). The poly-ɛ-caprolactone (PCL) units of the polymer form a hydrophobic membrane; the polyethylene glycol (PEG) units form a hydrophilic corona around the interior cavity of the PM. The polymer can be functionalized with a short peptide sequence such as TAT or Tet1 prior to formation. The peptide-functionalized polymer can be blended with unfunctionalized polymer and, upon formation, these peptides are present on the PM surface. (B) Shows the delivery location of the PMs via intact round window membrane (RWM) (topical RWM delivery and transtympanic injection) and cochleostomy.
Abbreviations: RWM, round window membrane; SGCs, spiral ganglion cells; TSF, tractus spiralis foraminosus.
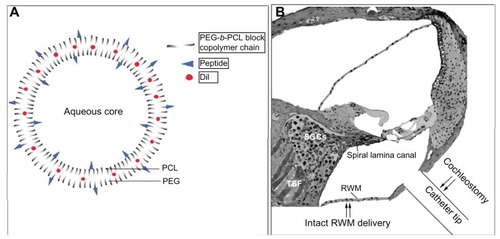
Figure 2 Illustration of the distribution of the polymersomes (PMs) after delivery using cochleostomy. In the cochlear nerve within the spiral lamina canal, the cochlear nerve in the tractus spiralis foraminosus region, and the spiral ligament (SL), the distribution patterns of Tet1-PMs, ScrTet1-PMs, and unlabelled PMs were different.
Notes: Arrowheads, the openings in ST that allowed the PMs to pass through.Citation19 White arrows with dashed lines, the possible routes of PM distribution in the inner ear.
Abbreviations: Tet1-PMs, Tet1-functionalized PMs; ScrTet1-PMs, scrambled Tet1 peptide modified PMs; RWM, round window membrane; SGCs, spiral ganglion cells; CN, cochlear nerve.
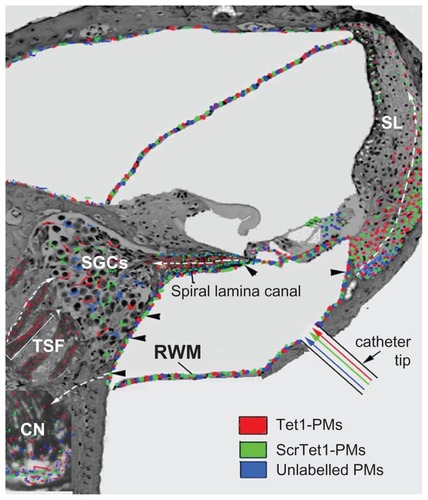
Figure 3 Distribution of Tet1-PMs, ScrTet1-PMs, and unlabelled polymersomes (PMs) in rat cochlea after administration via cochleostomy. All three types of PM were detected in the mesothelial cells of ST and SV (arrows), and spiral ganglion (SG) region (A–C). The fluorescence signal intensities of Tet1-PMs and ScrTet1-PMs were higher in the distal part (point 1 in A and B) than that in the proximal part (point 2 in A and B) of periphery processes of the spiral ganglion cells. Larger amount of ScrTet1- PMs and unlabelled PMs were detected in the epineurium (arrows and dashed lines in E and F) of peripheral processes than within the peripheral processes, whereas the Tet1-PMs were evenly distributed within the peripheral processes (D). D and E are longitudinal sections of peripheral processes. F is a transverse section of a peripheral processe.
Notes: Red: PMs. Blue: 4,6-diamidino-2-phenylindole. Gray (D–F): bright field channel. Scale bar in panels (A–C) = 10 μm. Scale bar in panels (D–F) = 100 μm.
Abbreviations: SV, scala vestibuli; ST, scala tympani; Tet1-PMs, Tet1-functionalized PMs; ScrTet1-PMs, scrambled Tet1 peptide modified PMs.
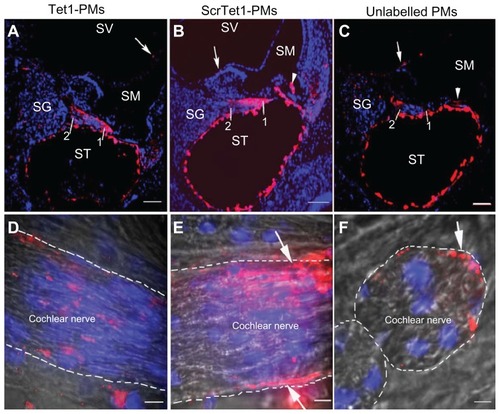
Figure 4 Location of Tet1-PMs, ScrTet1-PMs, and unlabelled polymersomes (PMs) in the cochlear nerve after administration via cochleostomy. Tet1-PMs (A) and ScrTet1- PMs (E) were detected in the spiral ganglion (SG) and the nerve fibers in the tractus spiralis foraminosus region of the CN. Only Tet1-PMs were detected in the tractus spiralis foraminosus region (arrows in A). Panels B–D show the co-localization of the PMs with NF-200 (arrows in panel B). The co-localized points are indicated by black dots in panels F (arrows), G and H (analyzed by ImageJ software). Arrowheads in panel B pointed to SGCs.
Notes: Red: PMs. Green: NF-200 immunostaining. Blue: 4,6-diamidino-2-phenylindole. Gray: bright field. Scale bar in A and E = 25 μm; scale bar in B–D = 10 μm.
Abbreviations: CN, cochlear nerve; NF-200, neurofilament 200; Tet1-PMs, Tet1-functionalized PMs; ScrTet1-PMs, scrambled Tet1 peptide modified PMs.
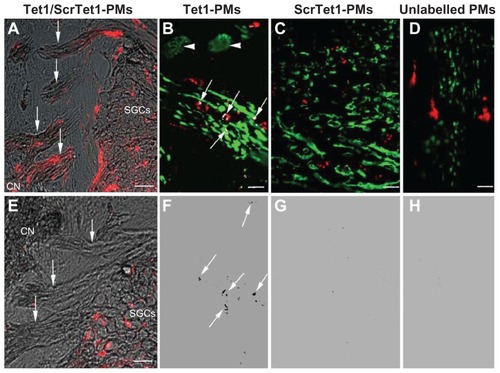
Figure 5 Distribution of Tet1-PMs, ScrTet1-PMs, and unlabelled polymersomes (PMs) in the spiral ganglion architectures after administration via cochleostomy. All three types of PM administrated by cochleostomy were located in the spiral ganglion satellite cells (SGSCs, arrows). PMs were not detected in the neuronal soma of the spiral ganglion cells.
Notes: *Nucleus of type I spiral ganglion cells. Red: PMs. Green: NF-200 or S100 immunostaining. Blue: 4,6-diamidino-2-phenylindole. Scale bar = 10 μm.
Abbreviations: Tet1-PMs, Tet1-functionalized PMs; ScrTet1-PMs, scrambled Tet1 peptide modified PMs.
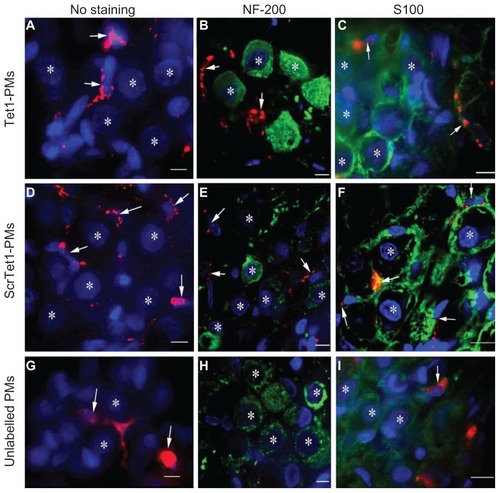
Figure 6 Distribution of polymersomes with and without Tet1 functionalization in the spiral ligament after administration via cochleostomy. A shows the Tet1-PMs detected in type II, III, and IV spiral ligament (SL) fibrocyte district. B shows the ScrTet1-PMs detected in type I, II, III, and IV spiral ligament fibrocyte district. C shows the unlabelled PMs in type III spiral ligament fibrocyte district.
Notes: Red: PMs. Blue: 4,6-diamidino-2-phenylindole. Scale bar = 50 μm.
Abbreviations: Tet1-PMs, Tet1-functionalized PMs; ScrTet1-PMs, scrambled Tet1 peptide modified PMs.