Figures & data
Figure 1 Ultraviolet-visible absorption spectra of reduced graphene oxide/titanium dioxide nanocomposites and graphene oxide.
Note: 120, 150, and 180 indicate the reaction temperature.
Abbreviations: GO, graphene oxide; RGO/TiO2, reduced graphene oxide/titanium dioxide nanocomposite.
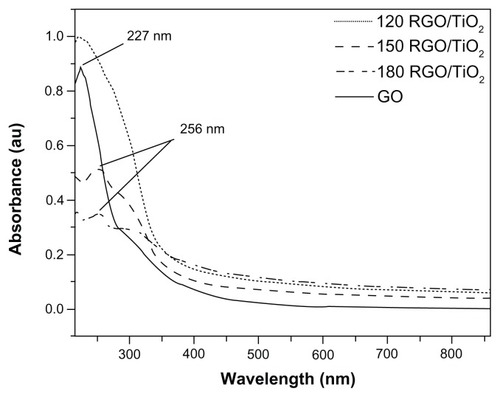
Figure 2 X-ray diffraction patterns of (A) graphite and graphene oxide and (B) reduced graphene oxide, titanium dioxide, and reduced graphene oxide/titanium dioxide nanocomposites.
Note: 120, 150, and 180 indicate the reaction temperature.
Abbreviations: GO, graphene oxide; RGO, reduced graphene oxide; RGO/TiO2, reduced graphene oxide/titanium dioxide nanocomposite; TiO2, titanium dioxide.
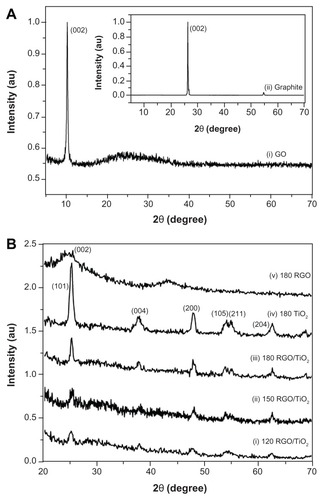
Figure 3 (A, C and E) Transmission electron micrograph and (B, D and F) histogram of reduced graphene oxide/titanium oxide nanocomposites at (A and B) 120°C, (C and D) 150°C, and (E and F) 180°C.
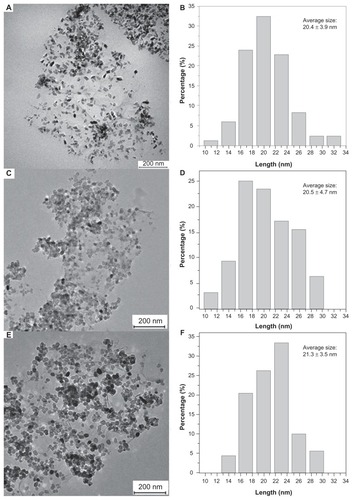
Figure 4 Transmission electron micrograph of reduced graphene oxide/titanium oxide nanocomposite synthesized without triethanolamine.
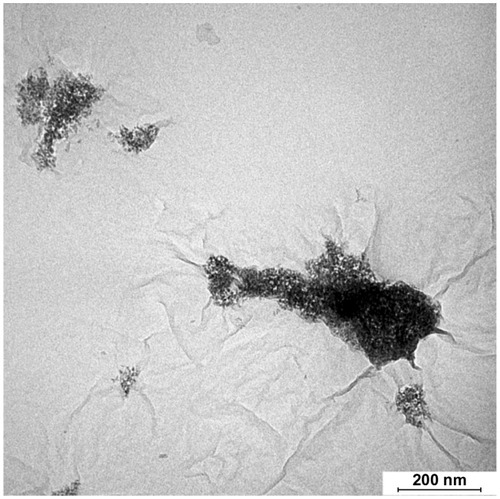
Figure 5 Thermogravimetric analysis curves of graphene oxide, reduced graphene oxide, titanium dioxide, and reduced graphene oxide/titanium dioxide nanocomposites.
Note: 120, 150, and 180 indicate the reaction temperature.
Abbreviations: GO, graphene oxide; RGO, reduced graphene oxide; RGO/TiO2, reduced graphene oxide/titanium dioxide nanocomposite; TiO2, titanium dioxide.
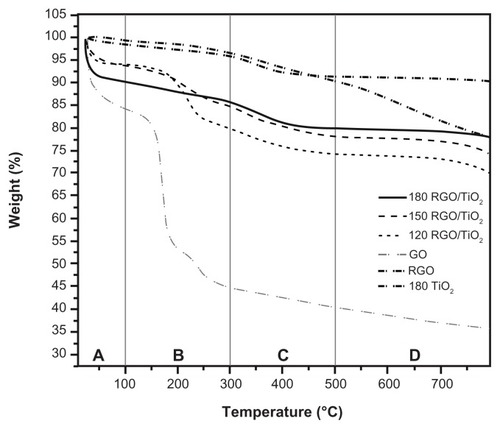
Figure 6 Schematic formation mechanism of the reduced graphene oxide/titanium dioxide nanocomposite. (A) Graphene oxide. (B) Electrostatic interaction between oxide functional groups of graphene oxide and titanium(IV) in the presence of triethanolamine. (C) Graphene decorated with titanium dioxide nanoparticles (filled circles) after hydrothermal treatment.
Abbreviations: H2O, water; TEA, triethanolamine; Ti4+, titanium(IV).
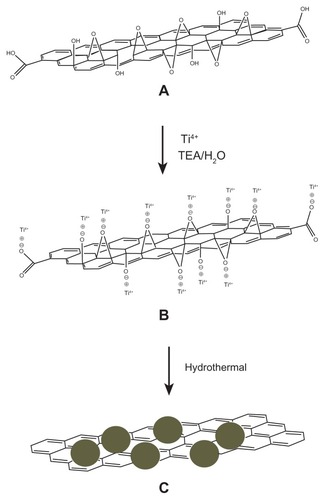
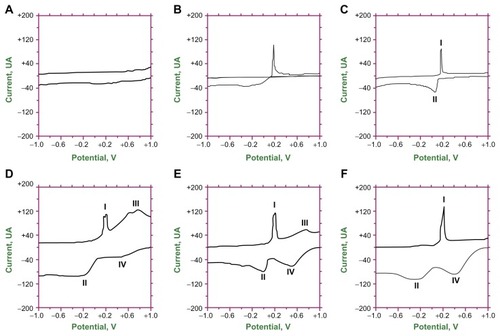
Figure 7 Electrochemical analysis of (A) bare glassy carbon electrode in potassium chloride supporting electrolyte, (B) bare glassy carbon electrode, (C) reduced graphene oxide-modified glassy carbon electrode, and reduced graphene oxide/titanium dioxide nanocomposite-modified glassy carbon electrode at (D) 150°C, (E) 180°C, and (F) 120°C in potassium chloride supporting electrolyte and mercury(II) ionic solution.