Figures & data
Figure 1 Images of (A) TEM image for solid silica nanoparticles, (B) TEM image of empty HMSNs, and (C) TEM image of silybin meglumine-loaded HMSNs.
Abbreviations: HMSNs, hollow-type mesoporous silica nanoparticles; TEM, transmission electron microscopy.
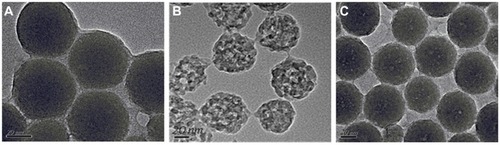
Figure 2 Nitrogen adsorption–desorption isotherms of solid silica nanoparticles (◆), empty HMSNs (▴), and silybin meglumine-loaded HMSNs (■). insert: line (A) represents empty HMSNs, line (B) represents silybin meglumine-loaded HMSNs, and line (C) represents solid silica nanoparticles.
Abbreviation: HMSNs, hollow-type mesoporous silica nanoparticles.
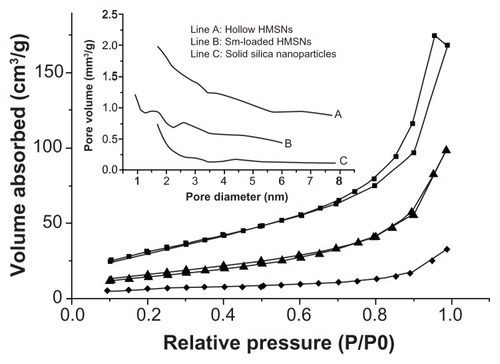
Figure 3 In vitro release curves of silybin from different formulations in various dissolution media. (A) In vitro release curves of silybin meglumine-loaded hollow-type mesoporous silica nanoparticles in artificial gastric juice (■) and artificial intestinal juice (▴); (B) in vitro release curves of silybin meglumine-loaded hollow-type mesoporous silica nanoparticles in Na2CO3 solutions with different concentrations [0.1 M (line A), 0.08 M (line B), 0.06 M (line C), and 0.01 M (line E)], and in 0.01 M NaOH solution (line D); (C) in vitro release curves of silybin meglumine-loaded solid silica nanoparticles in artificial gastric juice (■) and artificial intestinal juice (▴).
Note: Each datum point represents the mean ± standard deviation of three administrations.
![Figure 3 In vitro release curves of silybin from different formulations in various dissolution media. (A) In vitro release curves of silybin meglumine-loaded hollow-type mesoporous silica nanoparticles in artificial gastric juice (■) and artificial intestinal juice (▴); (B) in vitro release curves of silybin meglumine-loaded hollow-type mesoporous silica nanoparticles in Na2CO3 solutions with different concentrations [0.1 M (line A), 0.08 M (line B), 0.06 M (line C), and 0.01 M (line E)], and in 0.01 M NaOH solution (line D); (C) in vitro release curves of silybin meglumine-loaded solid silica nanoparticles in artificial gastric juice (■) and artificial intestinal juice (▴).Note: Each datum point represents the mean ± standard deviation of three administrations.](/cms/asset/511560be-a6ca-47f1-b29d-4451fc542419/dijn_a_28348_f0003_b.jpg)
Table 1 Mean plasma concentration-time data for silybin meglumine-loaded incorporated into hollow-type silica nanoparticles following a single dose
Figure 4 In vivo absorption fraction of silybin meglumine-loaded hollow-type mesoporous silica nanoparticles generated from the Wagner–Nelson method.
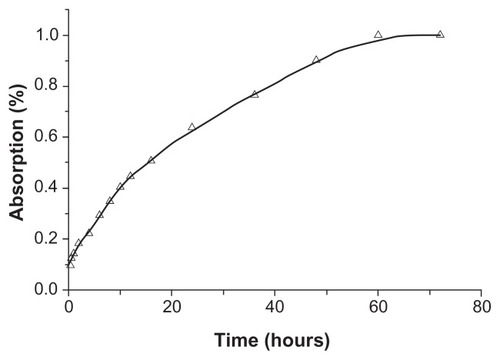
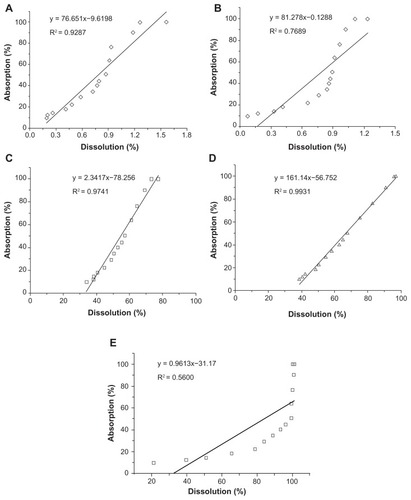
Figure 5 Correlations between in vitro dissolution fractions and in vivo absorption fractions. (A) IVIVC of silybin meglumine-loaded HMSNs in artificial gastric juice; (B) IVIVC of silybin meglumine-loaded HMSNs in artificial intestinal juice; (C) IVIVC of silybin meglumine-loaded HMSNs in 0.06 M Na2CO3 solution; (D) IVIVC of silybin meglumine-loaded HMSNs in 0.08 M Na2CO3 solution; and (E) IVIVC of silybin meglumine-loaded HMSNs in 0.1 M Na2CO3 solution.
Abbreviations: HMSNs, hollow-type mesoporous silica nanoparticles; IVIVC, in vitro–in vivo correlations.