Figures & data
Figure 1 Physicochemical characterization of doxorubicin-loaded magnetic Fe3O4 nanoparticles (DOX-MNPs): (A) transmission electron microscopy images of oleic acid-coated MNPs; (B) transmission electron microscopy images of DOX-MNPs; (C) size distribution of DOX-MNPs; (D) DOX-MNPs dispersed in aqueous solution could be attracted by an external magnetic field.
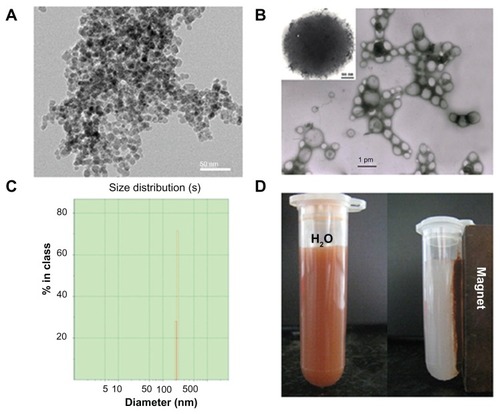
Figure 2 In vitro release profile of doxorubicin (DOX) from DOX-loaded magnetic Fe3O4 nanoparticles at pH 5.0 in acetate buffer and pH 7.4 in phosphate buffered saline.
Note: The results presented show the average from three measurements.
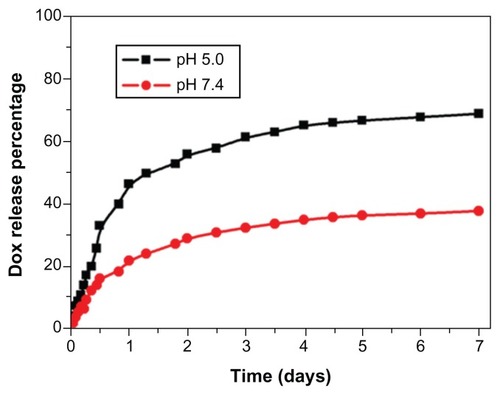
Figure 3 Observation of cellular uptake of doxorubicin-loaded magnetic Fe3O4 nanoparticles (DOX-MNPs) by different cell types after 30, 60, and 120 minutes of incubation under a fluorescence microscope. Overlaid images show nuclear staining with Hoechst 33258 (blue) and DOX-derived fluorescence (red). (A) Cellular uptake of free DOX and DOX-MNPs by Lewis lung carcinoma cells; (B) cellular uptake of DOX-MNPs by human osteosarcoma OS-732 cells and RAW 264.7 cells (murine-leukemic monocyte-macrophage cell line).
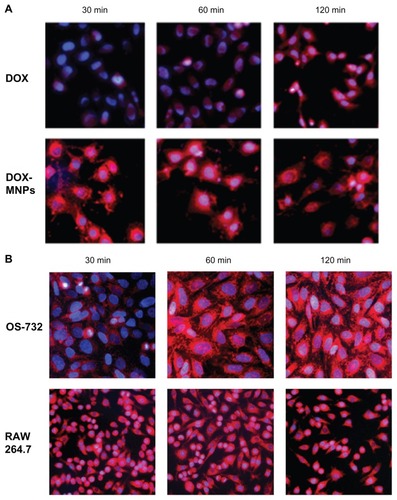
Figure 4 The apoptosis-induction effect of doxorubicin-loaded magnetic Fe3O4 nanoparticles (DOX-MNPs) in Lewis lung carcinoma cells (LLC). Fluorescein isothiocyanate– labeled Annexin V (Annexin V-FITC) and propidium (PI) double staining and flow cytometry were used to determine the proportion of live cells (Annexin V-FITC and PI double negative, bottom left quadrant), early apoptotic cells (Annexin V-FITC and PI negative, bottom right quadrant), late apoptotic cells (Annexin V-FITC and PI positive, top left quadrant), and necrotic cells (Annexin V-FITC and PI double positive, top right quadrant). (A) Representative histograms from flow cytometry of LLC cells treated with medium containing a series of concentrations of DOX-MNPs; (B) determination of live, apoptotic, and necrotic cells treated with a series of concentrations of DOX-MNPs; (C) representative histograms from flow cytometry of LLC cells treated with fresh medium containing free DOX (5 μg/mL) or equivalent concentrations of MNPs and DOX-MNPs; (D) determination of live, apoptotic and necrotic cells treated with free DOX (5 μg/mL) or equivalent concentrations of MNPs and DOX-MNPs.
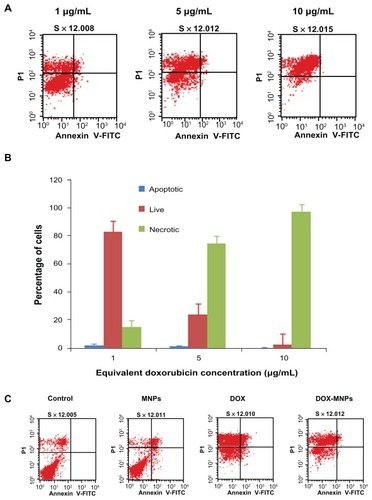
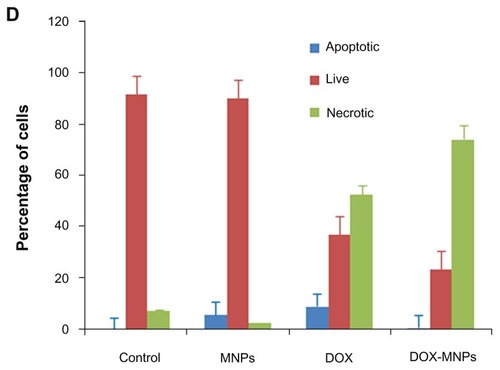
Figure 5 Antitumor effect of doxorubicin-loaded magnetic Fe3O4 nanoparticles (DOX-MNPs) in mice bearing subcutaneously established Lewis lung carcinoma.
Note: Tumor volume data given as mean plus or minus standard deviation.
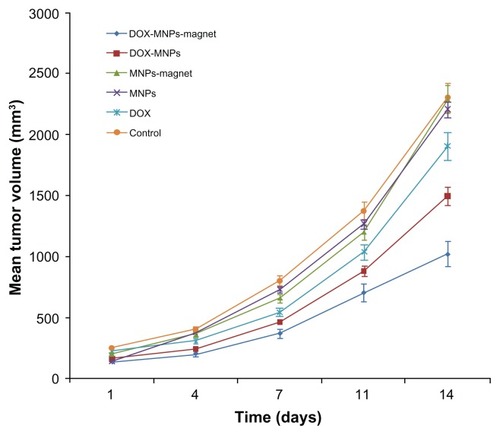
Table 1 Metastasis of Lewis lung carcinoma in mice after treatment
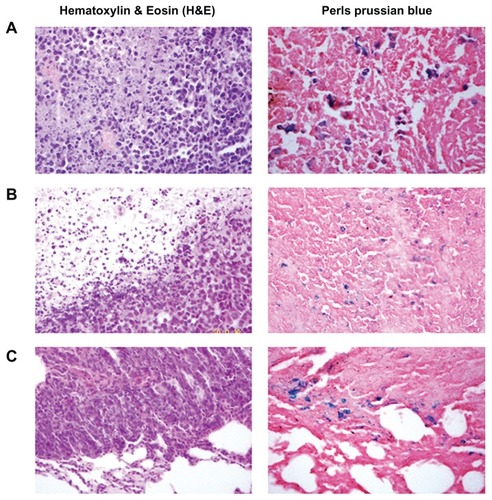
Figure 6 Histological analysis of the uptake of doxorubicin-loaded magnetic Fe3O4 nanoparticles (DOX-MNPs) in tumors (blue indicated the presence of iron): (A) marked accumulation of DOX-MNPs was observed in tumor cells with an external magnetic field; (B) less accumulation of DOX-MNPs in tumor cells without an external magnetic field; (C) accumulation of DOX-MNPs in lung metastasis of Lewis lung carcinoma.