Figures & data
Figure 1 A schematic representation of three models of the membrane, ie, the fluid mosaic model,Citation27 the membrane raft model,Citation28–Citation30 and the curvature-mediated lateral and orientational sorting model.Citation34,Citation35
Note: Violet color indicates orientational ordering of lipid molecules in the tubular portion.
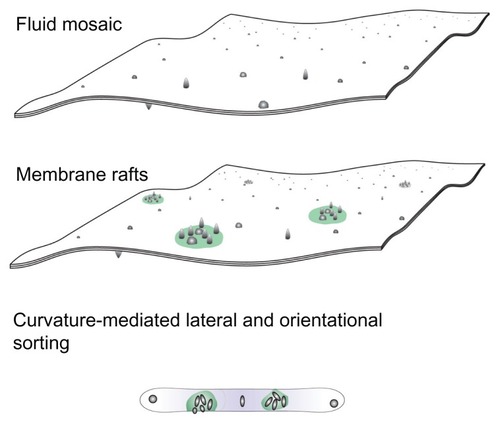
Figure 3 Free energy of a two-component nanovesicle as a function of the average mean curvature of the membrane for three interaction constants, ξ1/2kTRs2 (A) 0.001, (B) 0.020, and (C) 0.040. It was taken that ξ1 = ξ1* and ξ2 = ξ2*.
Notes: The values of other model parameters were ξ1/2kTRs2 = 0.001, h1,m = 2, d1,m = 2, h2,m = 0, d2,m = 0, M1,T = 0.1 MT, v = 0.5. Five characteristic equilibrium shapes obtained by solving the system of Euler-Lagrange equations subject to isotropic bending energy are also depicted at the corresponding 〈h〉 values.
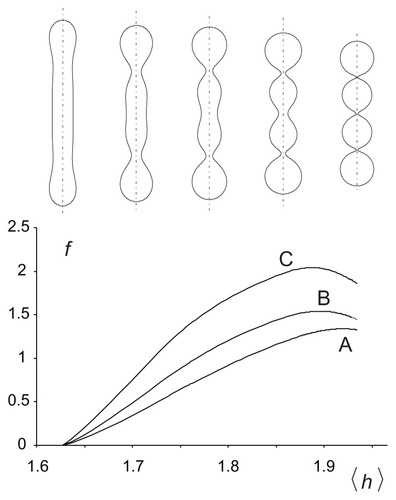
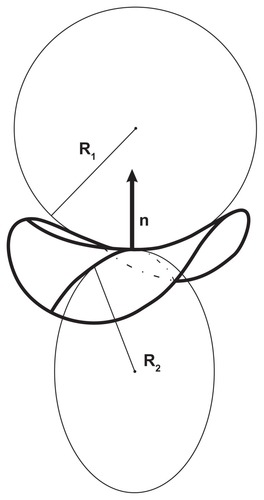
Figure 4 Calculated equilibrium configuration of three characteristic shapes of a two-component nanovesicle. It was taken that ξ1 = ξ1* and ξ2 = ξ2*,ξ2/2kTRs2 = 0.001, h1,m = 2, d1,m = 2, h2,m = 0, d2,m = 0, M1,T = 0.1 MT, v = 0.5. (D–I)ξ1/2kTRs2 = 0.04, (J–O) ξ1/2kTRs2 = 0.02, (P–U)ξ1/2kTR s2 = 0.001. (V–X) the characteristic shapes and (A–C) the respective invariants of the curvature tensor.
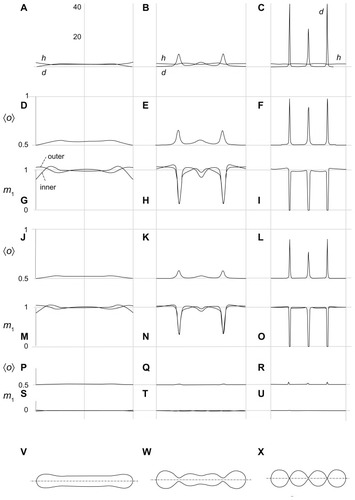
Figure 5 Nanobuds and nanovesicles of the erythrocyte membrane and nanotubules connecting T24 cancer cells. In erythrocytes, budding and vesiculation was induced by adding a detergent. The type of detergent determines the character of the nanobuds and nanovesicles. (A) Scanning electron micrograph of echinocyte budding induced by dodecylmaltoside, (B) transmission electron micrograph of isolated tubular nanovesicles induced by dodecylmaltoside, (C) scanning electron micrograph of the budding erythrocyte membrane, (D) scanning electron micrograph of echinocyte budding induced by dodecylzwittergent, (E) transmission electron micrograph of isolated spherical nanovesicles, (F) scanning electron micrograph of isolated spherical nanovesicles, (G) nanotubules with dilatations connecting urothelial cancer cells. (A, B, D, E, and G) reproduced with permission of Schara et alCitation63 and (C and F) reproduced from Sustar et al.Citation64
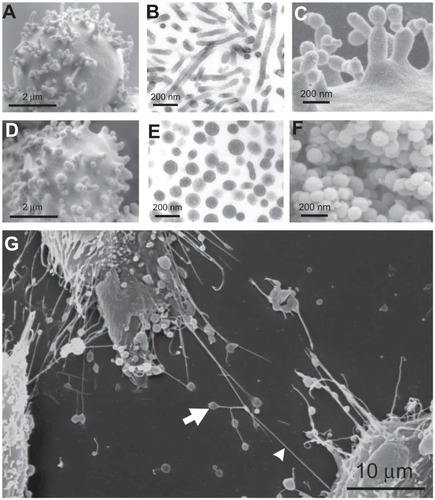
Figure 6 A (v, 〈h〉, 〈d〉) phase diagram with curves representing limit shapes (spheres, tubes, and tori) and characteristic shapes of microvesicles found in blood isolates (a sphere, a tube and a torus).
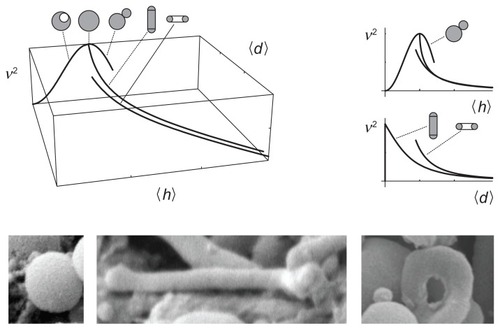
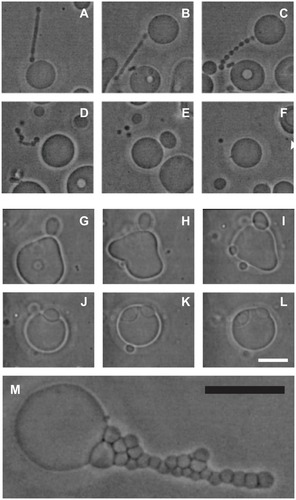
Figure 7 (A–F) Vesiculation of a giant phospholipid vesicle. After addition of phosphate-buffered saline to a suspension of vesicles, the tubular bud (A) exhibited undulations (B and C), detached itself from the mother vesicle (D), and decomposed into separate spherical vesicles (E), which were free to migrate away from the mother vesicle (F). (G–L) show suppression of vesiculation. When molecules which mediate attractive interaction between membranes (proteins dissolved in phosphate-buffered saline) were present in the solution, the bud (G and H) was attracted back to the mother membrane (I) where it remained bound to the surface of the mother vesicle (J–L). (M) Bead-like structures forming a long bud adhered to each other due to the mediating effect of added proteins dissolved in phosphate-buffered saline.
Notes: Bars represent 10 μm.
Reprinted from Urbanija et alCitation92 with the permission of Elsevier.