Figures & data
Figure 1 Schematic representation of MonY-Peg27(DTPA)-BN (MonY-BN).
Notes: MonY-BN molecule contains five components: a hydrophobic moiety with two C18 alkyl chains, a long polyoxoethilene spacer (Peg27), the DTPA chelating agent, a short linker (Ahoh), and the BN(7–14) peptide. The peptide sequence is reported using the three-letter amino acid code.
Abbreviations: BN, bombesin; DTPA, diethylenetriaminepentaacetate; PEG, polyethylene glycol.
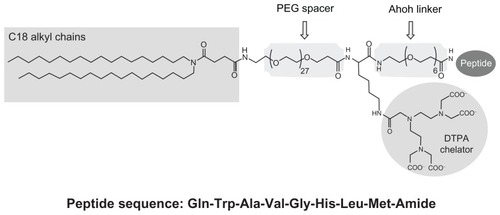
Figure 2 Synthetic procedure for obtaining MonY-BN by solid-phase methods.
Notes: The empty circle, ○, represents the solid support (Rink amide resin). The first step of the scheme reports the Fmoc removal by using DMF/Pip (70/30) mixture and the amino acid (Fmoc-aa-OH) coupling under standard SPPS conditions. This step is repeated eightfold to obtain the BN(7–14) sequence (reported according the single-letter amino acid code). Successively, solid-phase synthesis continues to bind on the peptide N-terminus to the other organic components. In the last step, cleavage (TFA/TIS/H2O) from the resin is performed to give a fully unprotected carboxamide product.
Abbreviations: BN, bombesin; DMF, N,N-dimethylformamide; DTPA, diethylenetriaminepentaacetate; PEG, polyethylene glycol; SPPS, solid-phase-peptide-synthesis; TFA, trifluoroacetic acid; TIS, tri-isopropylsylane.
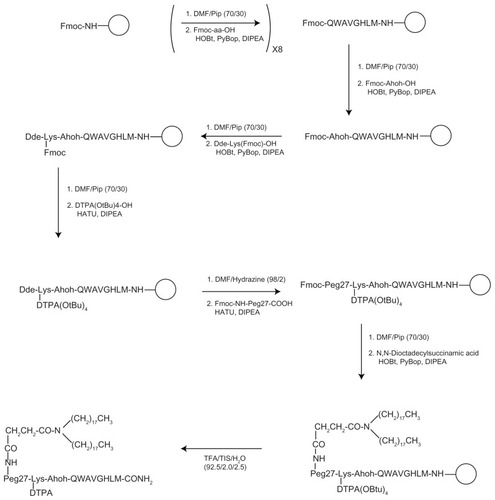
Table 1 Liposome characteristics
Figure 3 Binding assays of DSPC/MonY-BN and DSPC/(C18)2DTPA111In-radiolabeled liposomes on PC-3 cell line overexpressing the GRP at 37°C and 4°C at 1 hour.
Abbreviations: BN, bombesin; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; DTPA, diethylenetriaminepentaacetate; GRP, gastrin-releasing peptide.
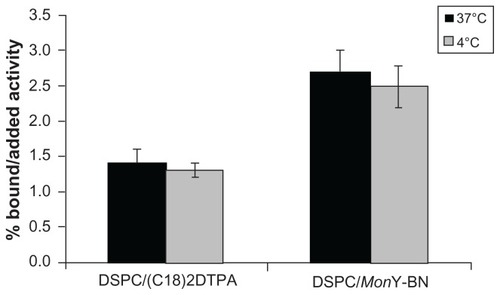
Figure 4 Cytotoxicity of liposomal Dox against human prostatic cancer cells.
Notes: PC-3 cells (8000 cells/well) were incubated with DSPC/MonY-BN/Dox, DSPC/Dox, and free Dox at 37°C (liposome formulations containing 500 or 1500 ng of lipids and 100 or 300 ng of Dox, respectively). Control experiments were performed using free Dox, and empty liposomes of DSPC/MonY or DSPC at the same experimental conditions. After 8 hours, the medium was removed, and after an additional 72 hours an MTS assay was performed. Data are expressed as percentage of control. Each value is the mean ± SEM of three experiments performed in triplicate.
Abbreviations: BN, bombesin; Dox, doxorubicin; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; SEM, standard error of the mean.
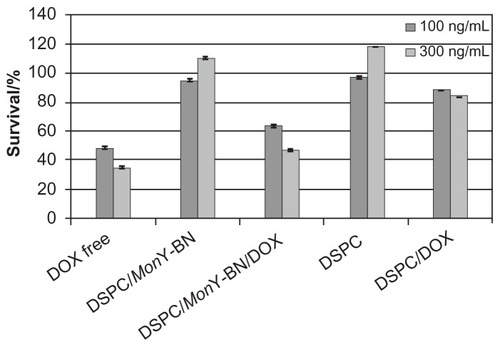
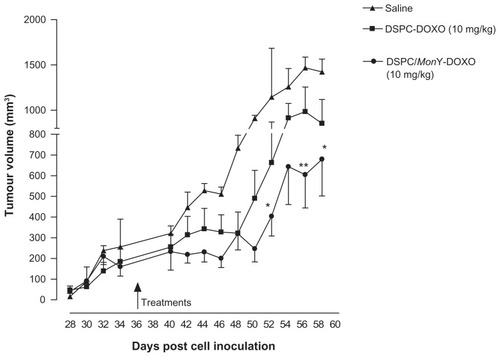
Figure 5 Comparative efficacy study in BALB/c nude mice bearing xenografts (PC-3) on tumor growth.
Notes: Treatment started approximately 5 weeks after cell implantation. Mice (n = 5) were administered intravenously with injection of 100 μL of liposome suspensions of DSPC/MonY-BN/Dox, or DSPC/Dox (1.0 × 10−2 M lipid concentration, at a dose of 10 mg of Dox/kg) or 100 μL of HBS buffer (control). Therapeutic efficacy was assessed by measuring tumor volume (mean ± SD) over time. Results were analyzed using two-way analysis of variance, followed by Bonferroni’s test. * denotes P < 0.05 and ** denotes P < 0.01 versus saline. DSPC/MonY-BN/Dox and DSPC/Dox produce, with respect to the saline solution, a TGI of 60% and 36%, respectively.
Abbreviations: BN, bombesin; Dox, doxorubicin; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; HBS, HEPES buffered saline; SD, standard deviation; TGI, tumor growth inhibition.