Figures & data
Figure 1 Synthesis procedures of ALA-p(His) by ring-opening polymerization of Bn-His-NCA, followed by deprotection: (A) PCl5, 1,4-dioxane; (B) N, N′-dimethylformamide; and (C) HBr/AcOH, TFA at 0°C.
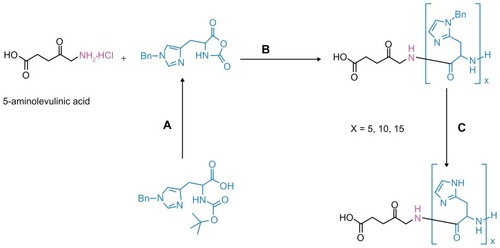
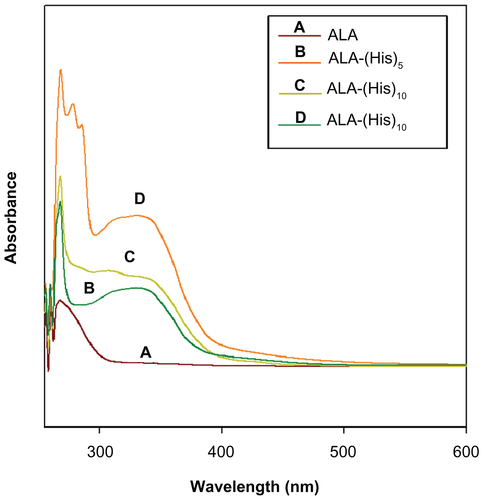
Figure 2 1H nuclear magnetic resonance spectra of ALA-p(His)15 in dimethyl-d6 sulfoxide at 25°C.
Abbreviation: ALA, 5-aminolevulinic acid.
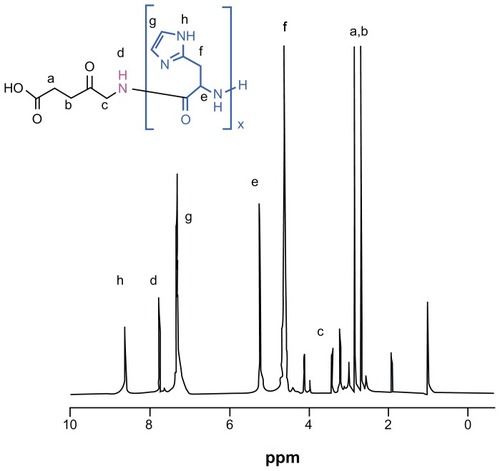
Table 1 Molecular properties of ALA and ALA-p(His)n molecules calculated using the density functional theory method in the aqueous phase
Figure 3 Geometry optimized structure of ALA and ALA-p(His)n, molecular orbital plots as well as Fukui active sites for electrophilic and nucleophilic attack on ALA and ALA-p(His)n molecules.
Note: The density functional theory and Fukui indices calculations were performed in the aqueous phase.
Abbreviations: ALA, 5-aminolevulinic acid; HOMO, highest occupied molecular orbital; LUMO, lowest occupied molecular orbital.
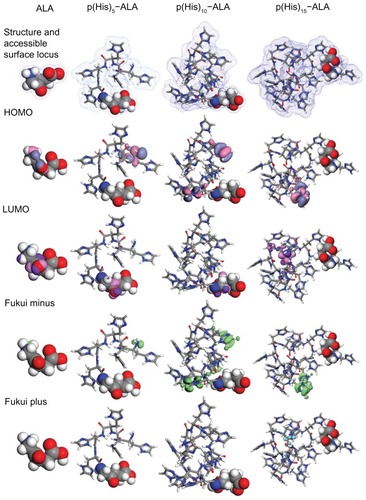
Figure 4 (A) Cytotoxicity of ALA and ALA-p(L-His) prodrugs in HEK293T cells. After 24 hours of starvation, the cells were treated with 0.1–1.0 mM of each ALA prodrug for 4 hours. Cell viability was determined by the MTT assay. (B) Cellular dark toxicity of ALA and ALA-p(L-His) prodrugs at 1 mM concentration in HCT116 cells at pH 6.8 and 7.4. After cultivation in a 96-well plate, the cells were treated with 1.0 mM of each ALA prodrug for 4 hours. Cell viability was determined by the MTT assay.
Abbreviations: ALA, 5-aminolevulinic acid; p(L-His), poly(L-histidine).
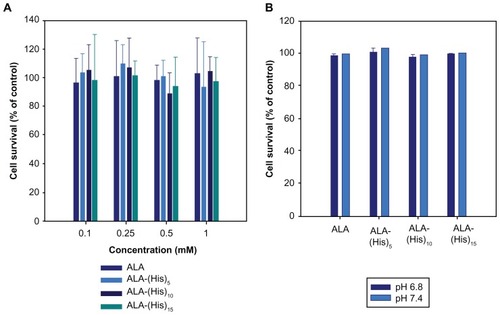
Figure 5 The photodynamic effect of ALA and ALA-p(L-His) prodrugs (1 mM concentration) on HCT116 cells at pH 7.4 and 6.8. 100 μL of serum-free medium containing 1.0 mM prodrugs was added to HCT116 cells following incubation for 4 hours.
Notes: The control was treated with serum-free medium in the absence of both ALA and p(L-His)-ALA. After irradiation at a dose of 1.0 J/cm2, the medium was removed and washed with phosphate-buffered saline. Next, a fresh 100 μL of RPMI with 10% phosphate-buffered saline was added and the cells were incubated for a further 24 hours. Cell phototoxicity was determined by MTT assay.
Abbreviations: ALA, 5-aminolevulinic acid; p(L-His), poly(L-histidine).
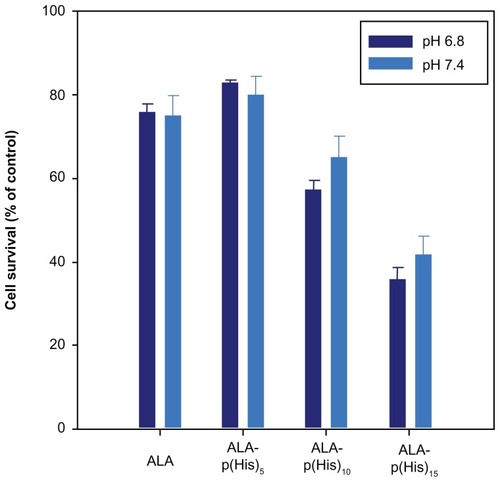
Figure 6 Fluorescence microscopic images of protoporphyrin IX accumulation from ALA and ALA-poly(L-histidine) prodrugs in HCT116 cells at pH 7.4 (A–D) and at pH 6.8 (A1–A4). HCT116 cells were treated with 1.0 mM ALA or poly(L-histidine)-ALA for 4 hours in serum-free media.
Note: The cells were fixed by 4% paraformaldehyde in phosphate-buffered saline and the cells were observed using a fluorescence microscope.
Abbreviation: ALA, 5-aminolevulinic acid.
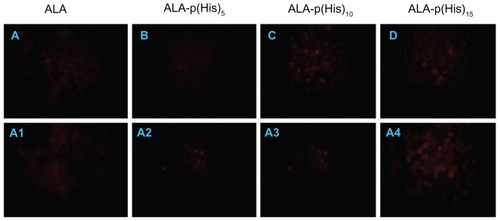
Figure 7 Apoptosis and necrosis induced by ALA or ALA-p(L-His)15 photodynamic therapy in HCT116 cells at pH 7.4 and 6.8. After prodrugs or nontreatment for 4 hours, HCT cells were illuminated at 635 nm. Cells were stained with FITC-annexin V (A) and propidium iodide (B), then analyzed using a flow cytometer.
Note: The number in the each square is the percentage of cells in the P2 or P3 population area of total cells.
Abbreviations: ALA, 5-aminolevulinic acid; p(L-His), poly(L-histidine).
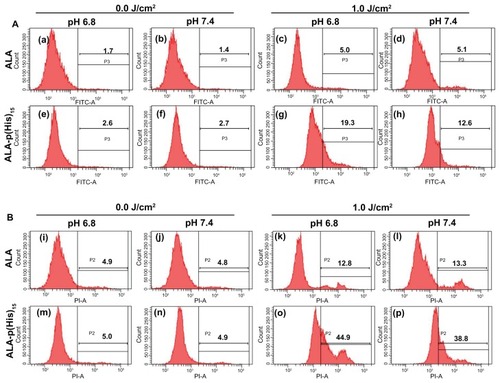
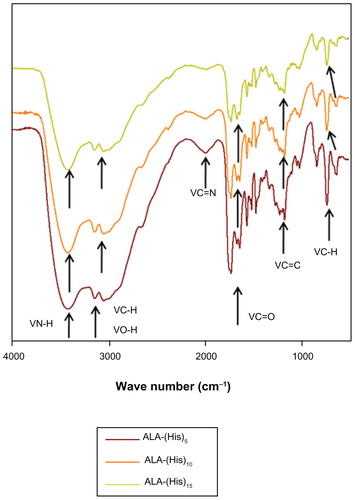
Figure S1 13C nuclear magnetic resonance spectra of ALA-p(His)15 in dimethyl-d6 sulfoxide at 25°C.
Abbreviation: ALA, 5-aminolevulinic acid.
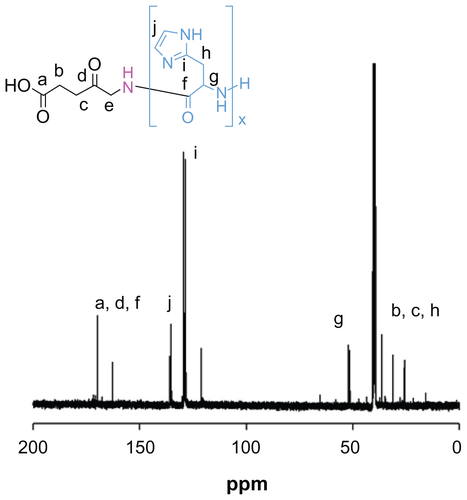
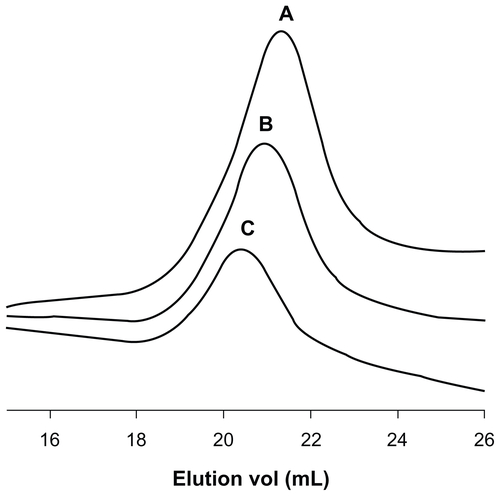
Figure S2 Gel permeation chromatography traces of (A) ALA-p(His)5, (B) ALA-p(His)10, and (C) ALA-p(His)15.
Note: Increment of molecular weight and monodispersity are clearly visible.
Abbreviation: ALA, 5-aminolevulinic acid.