Figures & data
Figure 1 Particles size distribution of CXCR4 siRNAI, II/dextran-spermin q3 = Fractional volume density distribution.
Notes: The size of the particles was calculated at a weight-mixing ratio of 1:5 (CXCR4 siRNAI, II to dextran-spermine). The average of size of particles was below 100 nm.
Abbreviation: q3*, density distribution by volume.
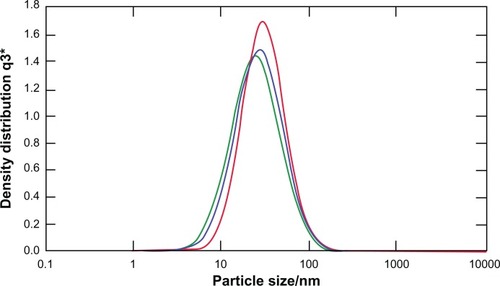
Figure 2 Zeta potential of CXCR4 siRNAI, II/dextran-spermine.
Notes: The zeta potential of particles was calculated at a weight-mixing ratio of 1:5 (CXCR4 siRNAI, II to dextran-spermine). The average of zeta potential was 39.7 ± 0.2 mV.
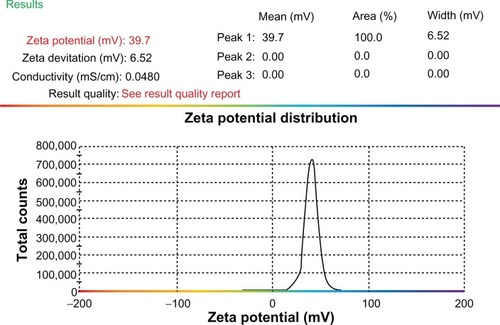
Figure 3 Melting curve for standard curve samples in real-time reverse transcription-polymerase chain reaction (RT-PCR) for β-actin endogeneous control: fluorescence melting curve obtained from real-time amplification of β-actin gene within the range of 72°C–97°C (A). Melting curve for standard curve samples in real-time RT-PCR for CXCR4 expression: fluorescence melting curve obtained from real-time amplification of CXCR4 gene within the range of 72°C–97°C (B). CXCR4 expression among groups A, B, C, D, and E: real-time RT-PCR was performed on the real-time PCR machine (tubes) Rotor-Gene 3000, and data analysis of CXCR4 and ß-actin expression was performed with the delta-delta Ct method. Standard curve was constructed with dilution (1:2) (C).
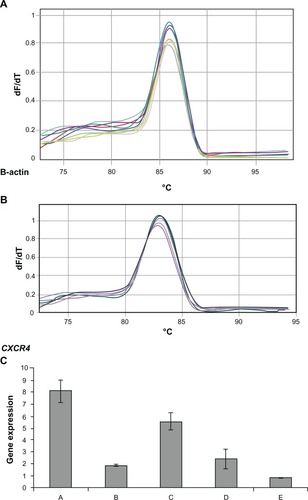
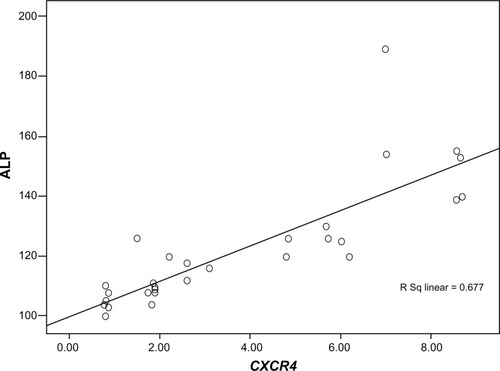
Figure 4 Serum alkaline phosphatase (ALP) level in mice groups A, B, C, D, E, and F after 30 days of treatment.
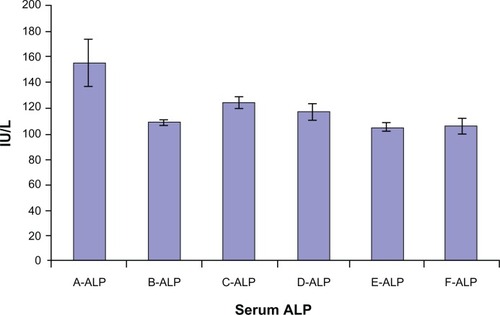
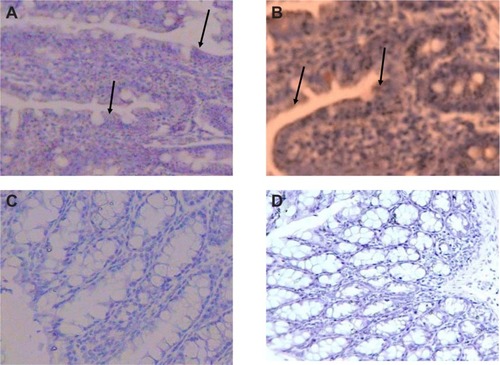
Figure 5 Immunohistochemical staining of the colon and distal ileum. Specimens of mouse colon and ileum were studied immunohistochemically for the presence of CXCR4 (integral membrane proteins). Sections labeled with goat antirabbit immunoglobulin G horseradish peroxides and developed with 3,3′-diaminobenzidine produced a brown color and were counterstained with hematoxylin (blue color). Immunohistochemical staining of the colon and distal ileum showed strong immunoreactivity in the colon and distal ileum in group A. Arrows show CXCR4 protein (magnification 40×) (A). Moderate immunoreactivity was detected in the colon and distal ileum in group D. Arrows show CXCR4 protein (magnification 40×) (B). Faint immunoreactivity was detected in the colon in group E (magnification 40×) (C). Faint to negative immunoreactivity was detected in the colon in group F (magnification 40×) (D).