Figures & data
Figure 1 Schematic illustration of the fabrication process.
Note: After modification with PAH/PSS, diatomite particles were negatively charged and could adsorb positively charged nano-ZrO2.
Abbreviations: PAH, poly(allylamine hydrochloride); PSS, poly(sodium 4-styrenesulfonate).
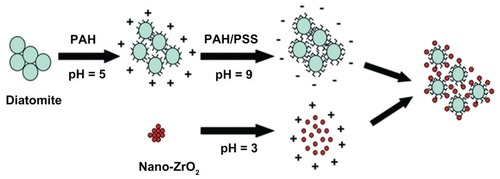
Figure 2 Size distribution of diatomite particles. (A) Before modification, the size distribution of diatomite particles spanned from 0.2 to 90 μm and d(0.5) was 16.42 μm. (B) After PAH/PSS modification, the diameter shifted to the left, size distribution narrowed (from 0.12 to 35 μm) and d(0.5) decreased to 0.42 μm.
Abbreviations: PAH, poly(allylamine hydrochloride); PSS, poly(sodium 4-styrenesulfonate).
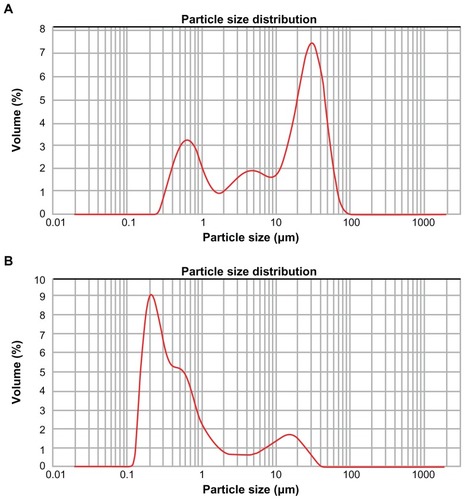
Figure 3 X-ray photoelectron spectroscopy spectrum of diatomite before and after modification. Before modification (A), Si2p and O1s were detected at 102.66 eV(2p) and 532.05 eV(1s); after modification by PAH (B), a clear N1s signal was present at 399.55 eV(1s), corresponding to the amine of PAH.
Note: After modification with PAH/PSS, the spectrum showed characteristic peaks of S2p at 168 eV(S2p), assigned to the sulfur of the sulfonate group of PSS.
Abbreviations: PAH, poly(allylamine hydrochloride); PSS, poly(sodium 4-styrenesulfonate).
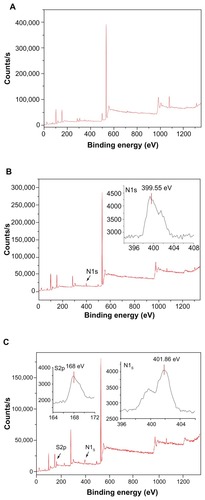
Figure 4 Scanning electron microscopy images of diatomite-based particles containing 30 wt% nano-ZrO2. (A) Image shows agglomeration of unmodified diatomite particles containing agglomerated nano-ZrO2. After modification (B), the diatomite particles are more uniformly distributed and nano-ZrO2 particles are adsorbed.
Note: A typical structure is circled in (B).
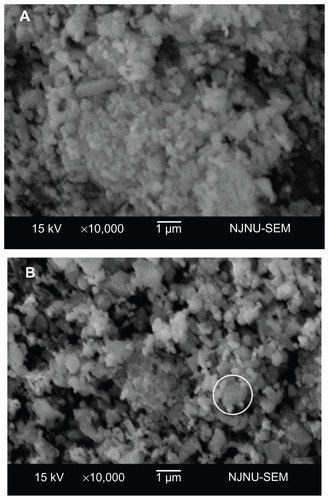
Figure 5 Scanning electron microscopy analysis of sections of the sintered ceramics. (A and B) show sintered unmodified ceramics containing 30 wt% nano-ZrO2 (U30Z), with a large volume of irregular pores. (C and D) show sintered modified ceramics containing 30 wt% nano-ZrO2 (M30Z). Compared with those shown in (A and B), these ceramics exhibit high density with low porosity.
Note: The element of a typical structure was detected by EDS, confirming that it was composed of diatomite with adsorbed nano-ZrO2 particles.
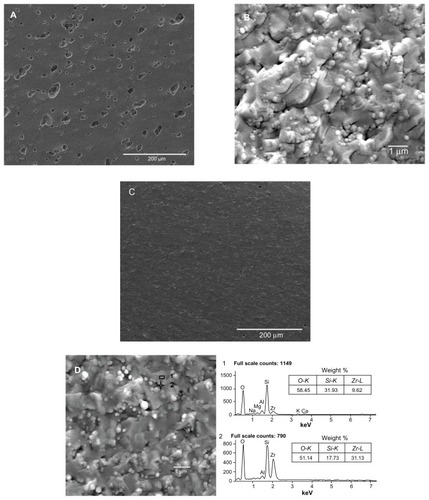
Figure 6 Open porosity of unmodified and modified diatomite-based nanocomposite sintered bodies containing 0, 20, 25, 30, and 35 wt% nano-ZrO2.
Note: Open porosity declined after modification.
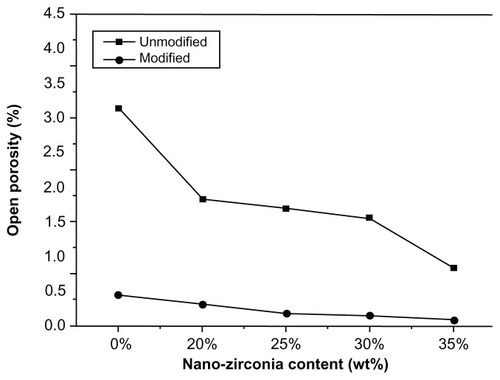
Figure 7 Mechanical properties of unmodified and modified diatomite-based nanocomposite sintered bodies containing 0, 20, 25, 30, and 35 wt% nano-ZrO2. (A) Flexural strength, (B) Vickers hardness, and (C) fracture toughness.
Note: Generally, the modified groups had better mechanical properties than the unmodified ones.
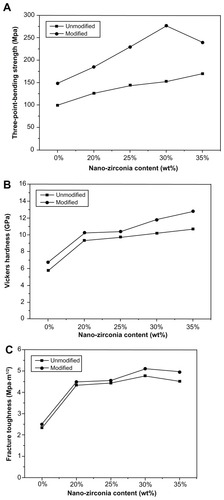
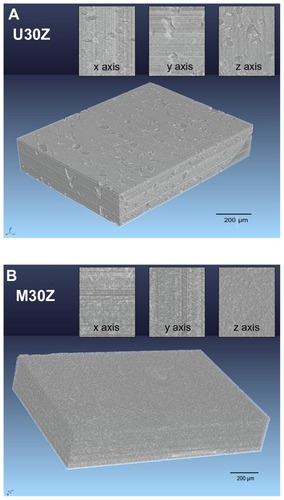
Figure 8 Three-dimensional phase-contrast synchrotron X-ray microtomography representations of U30Z (A) and M30Z (B) ceramics and their random cross-section images from x, y, and z axes. A large number of holes are distributed in the sintered body of U30Z (A), while in M30Z, no pores are visible to the naked eye from the obtained images (B), indicating that the modification of ceramic powders by the layer-by-layer technique can significantly increase the density of the sintered body.