Figures & data
Figure 1 Stem cell differentiation mediated by bionanomaterials with applications in regenerative medicine.
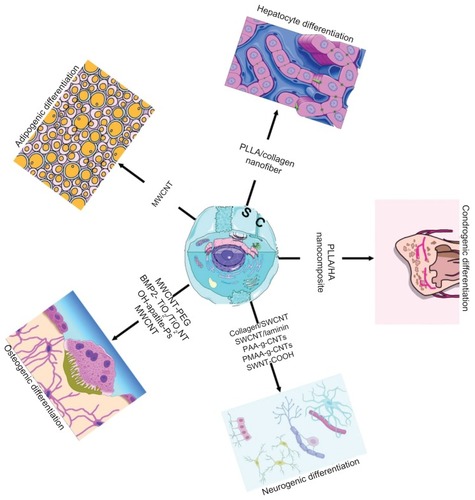
Figure 2 Osteogenic differentiation of human mesenchymal stem cells characterized by calcium deposition. The human mesenchymal stem cells were cultured with the materials as listed in the column.
Notes: Data are presented as the mean ± standard error of the mean (n = 3). *P < 0.05 compared with glass and PSTC (8 and 9); **P < 0.05 compared with PLGA-P and PLGA (5 and 6); #P < 0.05 compared with DIF-7c peptide (7). Reprinted with permission Yang et al.Citation6
Abbreviations: MSC, mesenchymal stem cells; HA, hydroxyapatite; PLGA, polylactide-co-glycolide; P, peptide; Ps, peptide loaded by aminosilane chemistry; HA-Ps, nano-HA loaded with peptide using aminosilane chemistry; PLGA-P, PLGA scaffold loaded with peptide; PTSC, polystyrene tissue culture plate; DIF-7c, peptide-derived short peptide.
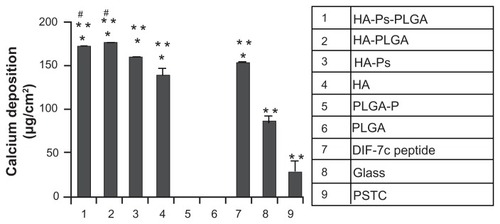
Table 1 Summary of the literature reports indicating applications of some nanomaterials in stem cell differentiation and proliferation
Figure 3 In vitro and in vivo double differentiation of human mesenchymal stem cells (EGFP negative) seeded on scaffolds where one side was coated with TransITTKO nanoparticles containing TRIB2-targeted siRNA (#1) and the other side with TransIT-TKO nanoparticles containing BCL2L2-targeted siRNA (#1). (A) In vitro double differentiation. The dual-coated scaffolds were seeded with human mesenchymal stem cells and cultured for 2 days in maintenance medium and 8 days in complex medium. The sides were then separated and for each side the ratios of the mRNA levels of PPARγ2, RUNX2, AP2, ADN, OC and alkaline phosphatase relative to the mRNA level of B2M were determined. The ratios of the marker expression between the side with the TRIB2-targeted siRNA (#1) and the side with the BCL2L2-targeted siRNA (#1) were characterized and plotted. Averages are graphed with error bars representing standard deviation (n = 3 or 4). The y-axis is logarithmic and the markers that were upregulated on the side coated with TRIB2-targeted siRNA compared with the side coated with BCL2L2-targeted siRNA will have values above 1 and vice versa. (B–D) In vivo differentiation on a dual-coated scaffold. The dual-coated scaffolds were seeded with human mesenchymal stem cells for 16 hours in maintenance medium. They were then implanted subcutaneously in non-obese diabetic/severe combined immunodeficient mice for 2 weeks, after which they were surgically removed and studied by histology. Whole scaffold sections were stained with (B) hematoxylin and eosin, (C) Sirius red, and (D) von Kossa. Mosaic pictures of whole scaffold sections at × 10 magnification are displayed with the BCL2L2 siRNA-coated side in the top of the pictures, host tissue can be seen surrounding the implant. On the Sirius red-stained section, collagen deposition appears red and birefringence appears orange. Images in (B) and (C) are 7.6 mm wide and 8.4 mm high, and (D) is 7.6 mm wide and 6.5 mm high. Reprinted with permission from Andersen et al.Citation69
Abbreviations: ADN, adiponectin; ALP, alkaline phosphatase; AP2, apolipoprotein 2; B2M, β-2 microglobulin; COL1, collagen type I; EGFP, enhanced green fluorescent protein; LPL, lipoprotein lipase; OC, osteocalcin; PPARγ2, peroxisome proliferator-activated receptor-γ isoform 2; siRNA, small-interfering RNA.
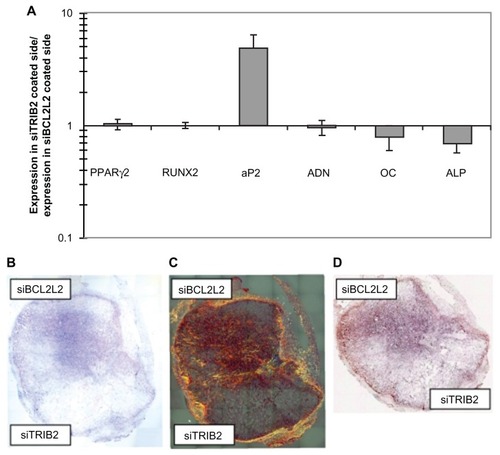
Figure 4 Micrograph assessing NSC cell adhesion and differentiation 72 hours after initial seeding on (A) laminin-coated glass slides and on 10 bilayered thin SWCNT-laminin films that were (B) used as is or (C) heated at 300°C for 10 minutes. (D) Distance of outgrowth from neurospheres after 24 hours (yellow), 48 hours (red), 72 hours (blue), and 120 hours (green) on laminin-coated slides and heat-treated SWCNT-laminin film on slide. (E) Live-dead viability assay on seeded cells where live cells are stained green and dead cells are red.
Note: Scale bars are 200 μm. Reprinted with permission Kam NWS, Jan E, Kotov NA. Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett. 2008;9(1):273–278. Copyright 2008 American Chemical Society.
Abbreviations: NSC, neural stem cells; SWCNTs, single-walled carbon nanotubes.
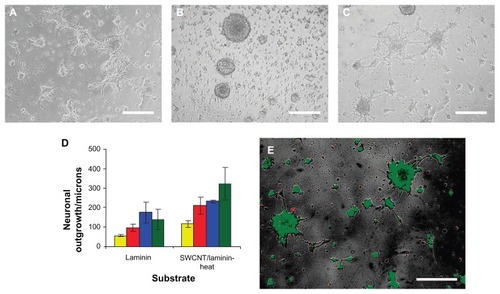
Figure 5 Atomic force microscopy characterization (amplitude images) of the surface structures of gelatin (A), collagen (B), and collagen/carbon nanotubes (C) matrices prepared under the cell culture conditions.
Note: Image size, 8.5 × 8.5 μm2, inset 2 × 2 μm2. Reprinted with permission from Sridharan et al.Citation89
