Figures & data
Figure 1 Reaction scheme for the covalent coupling of chitosan-TGA to a maleimide-functionalized phospholipid to form a stable thioether bond.
Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; TGA, thioglycolic acid.
![Figure 1 Reaction scheme for the covalent coupling of chitosan-TGA to a maleimide-functionalized phospholipid to form a stable thioether bond.Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; TGA, thioglycolic acid.](/cms/asset/3c0e8b41-5526-4f4c-b283-b5d85b5f0515/dijn_a_29980_f0001_c.jpg)
Figure 2 Falling Liquid Film technique to measure the mucoadhesion of coated and uncoated liposomes.
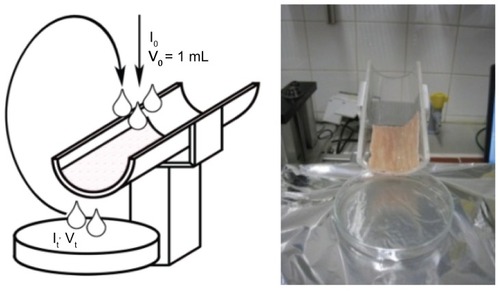
Figure 3 Particle size after the addition of different amounts of polymers (given by the molar ratio of SH-groups of the polymer to maleimide groups of the liposome) to POPC liposomes (○) and POPC/DOPE-MCC liposomes (■).
Note: Indicated values are means ± standard deviation of at least three measurements.
Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N- [4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoylphosphatidylcholine; TC, chitosan-thioglycolic acid.
![Figure 3 Particle size after the addition of different amounts of polymers (given by the molar ratio of SH-groups of the polymer to maleimide groups of the liposome) to POPC liposomes (○) and POPC/DOPE-MCC liposomes (■).Note: Indicated values are means ± standard deviation of at least three measurements.Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N- [4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoylphosphatidylcholine; TC, chitosan-thioglycolic acid.](/cms/asset/38560174-e5e4-4aee-8a60-dc27543baa44/dijn_a_29980_f0003_b.jpg)
Figure 4 Zeta potential after the addition of different amounts of polymers (given by the molar ratio of SH-groups of the polymer to maleimide groups of the liposome) to POPC liposomes (○) and POPC/DOPE-MCC liposomes (■).
Note: Indicated values are means ± standard deviation of at least three measurements.
Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine- N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoylphosphatidylcholine; TC, chitosan-thioglycolic acid.
![Figure 4 Zeta potential after the addition of different amounts of polymers (given by the molar ratio of SH-groups of the polymer to maleimide groups of the liposome) to POPC liposomes (○) and POPC/DOPE-MCC liposomes (■).Note: Indicated values are means ± standard deviation of at least three measurements.Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine- N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoylphosphatidylcholine; TC, chitosan-thioglycolic acid.](/cms/asset/55ba17b0-239c-4e8c-beba-da76863e59fb/dijn_a_29980_f0004_c.jpg)
Table 1 Amount of SH-groups after coupling
Figure 5 Transmission electron micrographs with negative staining technique of (A) uncoated POPC/DOPE-MCC liposomes and (B) POPC/DOPE-MCC liposomes coated with chitosan-TGA (4:1 molar ratio of SH-groups to maleimide groups).
Notes: Magnification: 30,000×. Scale bar indicates 200 nm.
Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.
![Figure 5 Transmission electron micrographs with negative staining technique of (A) uncoated POPC/DOPE-MCC liposomes and (B) POPC/DOPE-MCC liposomes coated with chitosan-TGA (4:1 molar ratio of SH-groups to maleimide groups).Notes: Magnification: 30,000×. Scale bar indicates 200 nm.Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.](/cms/asset/d2502c97-b7fd-4af7-a0e5-1827e0bf2d09/dijn_a_29980_f0005_b.jpg)
Figure 6 Transmission electron micrographs using freeze fracturing of (A) uncoated POPC/DOPE-MCC liposomes and (B) POPC/DOPE-MCC liposomes coated with chitosan-TGA (4:1 molar ratio of SH-groups to maleimide groups).
Notes: Arrows indicate the polymer coat. Magnification: 30,000×. Scale bar indicates 200 nm.
Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.
![Figure 6 Transmission electron micrographs using freeze fracturing of (A) uncoated POPC/DOPE-MCC liposomes and (B) POPC/DOPE-MCC liposomes coated with chitosan-TGA (4:1 molar ratio of SH-groups to maleimide groups).Notes: Arrows indicate the polymer coat. Magnification: 30,000×. Scale bar indicates 200 nm.Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.](/cms/asset/13529686-d0d0-4b4d-b95d-3351decd6a32/dijn_a_29980_f0006_b.jpg)
Figure 7 Release of ANTS/DPX at predetermined time points from uncoated POPC/DOPE-MCC liposomes (○) and coated POPC/DOPE-MCC liposomes (4:1 molar ratio of SH-groups to maleimide groups) (■) in simulated gastric fluid (A) and simulated intestinal fluid (B).
Note: Each point represents the mean value of two different determinations.
Abbreviations: ANTS, anionic fluorophore 8-aminonaphthalene-1,3,6-trisulfonic acid; DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl) cyclohexane-carboxamide]; DPX, p-xylene-bis-pyridinium bromide; POPC, Palmitoyl-oleoyl-phosphatidylcholine.
![Figure 7 Release of ANTS/DPX at predetermined time points from uncoated POPC/DOPE-MCC liposomes (○) and coated POPC/DOPE-MCC liposomes (4:1 molar ratio of SH-groups to maleimide groups) (■) in simulated gastric fluid (A) and simulated intestinal fluid (B).Note: Each point represents the mean value of two different determinations.Abbreviations: ANTS, anionic fluorophore 8-aminonaphthalene-1,3,6-trisulfonic acid; DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl) cyclohexane-carboxamide]; DPX, p-xylene-bis-pyridinium bromide; POPC, Palmitoyl-oleoyl-phosphatidylcholine.](/cms/asset/9a6e8ec3-42d0-421b-9c2b-718dd617606e/dijn_a_29980_f0007_b.jpg)
Figure 8 Release of FITC-calcitonin at predetermined time points from uncoated POPC/DOPE-MCC liposomes (○) and coated POPC/DOPE-MCC liposomes (4:1 molar ratio of SH-groups to maleimide groups) (■) in simulated gastric fluid (A) and simulated intestinal fluid (B).
Note: Results are means ± standard deviation (n = 3).
Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine.
![Figure 8 Release of FITC-calcitonin at predetermined time points from uncoated POPC/DOPE-MCC liposomes (○) and coated POPC/DOPE-MCC liposomes (4:1 molar ratio of SH-groups to maleimide groups) (■) in simulated gastric fluid (A) and simulated intestinal fluid (B).Note: Results are means ± standard deviation (n = 3).Abbreviations: DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine.](/cms/asset/b321fc51-578a-4584-af5c-f782191b0c76/dijn_a_29980_f0008_c.jpg)
Table 2 Percentage of uncoated/coated liposomes bound to the tissue after using the falling liquid film technique
Figure 9 Histological sections of porcine small intestine. (A) untreated intestinal tissue (negative control), (B) tissue treated with POPC/DOPE-Liss Rhod liposomes and (C) with POPC/DOPE-Liss Rhod/DOPE-MCC liposomes coated with chitosan-TGA (molar ratio of SH-groups to maleimide-groups: 4:1).
Abbreviations: DOPE-Liss Rhod, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl); DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.
![Figure 9 Histological sections of porcine small intestine. (A) untreated intestinal tissue (negative control), (B) tissue treated with POPC/DOPE-Liss Rhod liposomes and (C) with POPC/DOPE-Liss Rhod/DOPE-MCC liposomes coated with chitosan-TGA (molar ratio of SH-groups to maleimide-groups: 4:1).Abbreviations: DOPE-Liss Rhod, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl); DOPE-MCC, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl)cyclohexane-carboxamide]; POPC, Palmitoyl-oleoyl-phosphatidylcholine; TGA, thioglycolic acid.](/cms/asset/b0648607-032f-453c-a5d7-6d94fe159fd6/dijn_a_29980_f0009_c.jpg)