Figures & data
Table 1 Entrapment efficiencies of 5-fluorouracil as a function of different initial drug concentrations and nanoaggregates’ average diameters
Figure 1 Scanning electron micrograph (SEM) images for different magnetic nanoaggregate formulations. Effect of polymeric composition on morphology of nanoaggregates: (A) 0.5 mmol block copolymer and 0 wt% beta-cyclodextrin; (B) 3 mmol block copolymer and 0 wt% beta-cyclodextrin; (C) 3 mmol block copolymer and 5 wt% beta-cyclodextrin; and (D) 3 mmol block copolymer and 25 wt% beta-cyclodextrin.
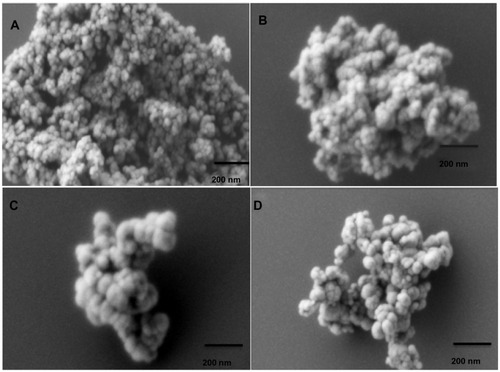
Figure 2 Effect of block copolymer concentrations on the average primary and aggregated particle diameters.
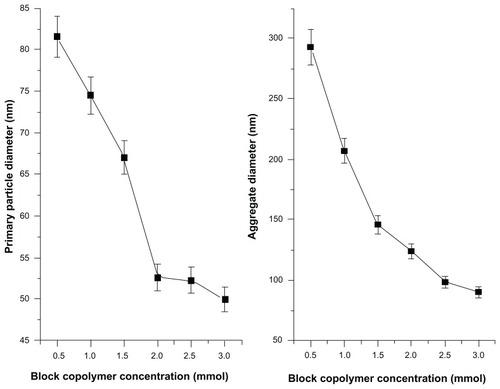
Figure 3 X-ray diffraction profiles of magnetic nanoaggregates as a function of block copolymer concentration. (A) 0.5 mmol; and (B) 3 mmol of block copolymer (Pluronic F-68).
Abbreviation: CPS, counts per second.
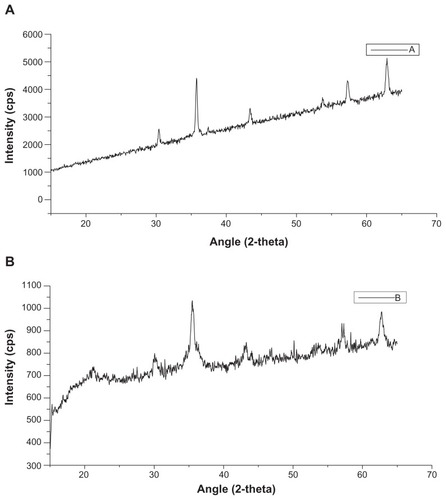
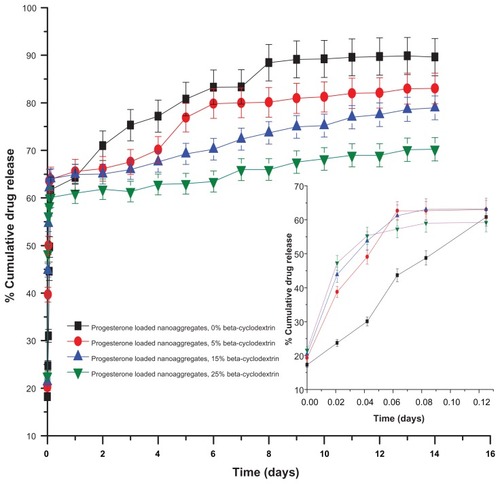
Figure 4 FTIR spectrum of uncoated magnetic nanoaggregates.
Abbreviation: FTIR, fourier transform infrared spectroscopy.
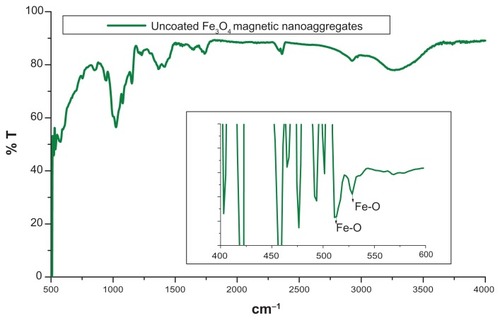
Figure 5 FTIR spectra of different polymer coated magnetic nanoaggregates.
Abbreviation: %T, % Transmittance.
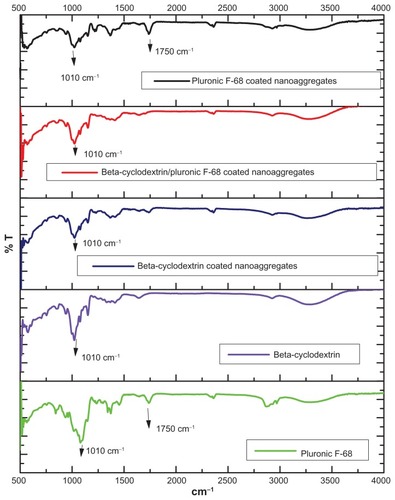
Figure 6 Room temperature (300 K) magnetization curves of magnetic nanoaggregates prepared with 3 mmol of block copolymer and 5 wt% beta-cyclodextrin compared to magnetic nanoparticles prepared by conventional method.
Abbreviation: emu, electro-magnetic unit.
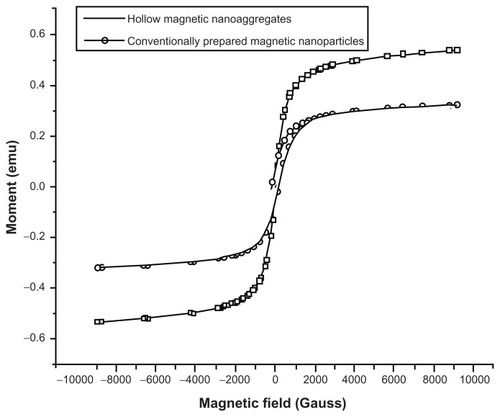
Table 2 Estimated Peppas parameters as a function of drug loading percentages and loading techniques
Figure 8 Effect of beta-cyclodextrin mass fraction on the release of progesterone samples loaded by freeze-drying.
Scheme 1 Chemical structures of beta-cyclodextrin, polypropylene oxide/polypropylene oxide block copolymer (Pluronic F-68, HO (C2H4O)n(C3H6O)m(C2H4O)n OH, m = 80 and n = 27) and the two encapsulated drugs: progesterone and 5-fluorouracil.
Table 3 Release parameters for mathematical modeling of progesterone-loaded magnetic nanoaggregates
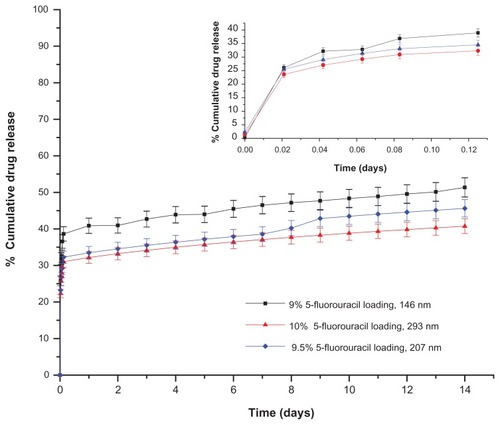
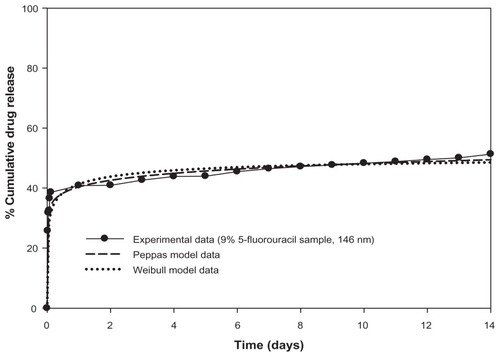
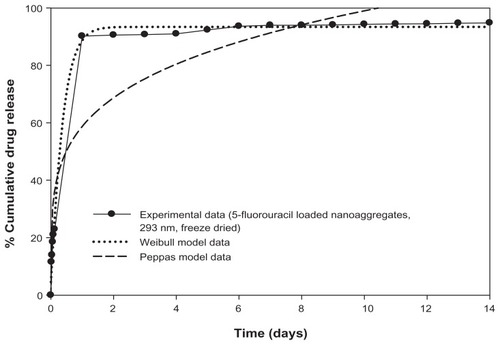
Figure S1 Drug release profiles of 5-fluorouracil-loaded nanoaggregates prepared by in-situ loading method.
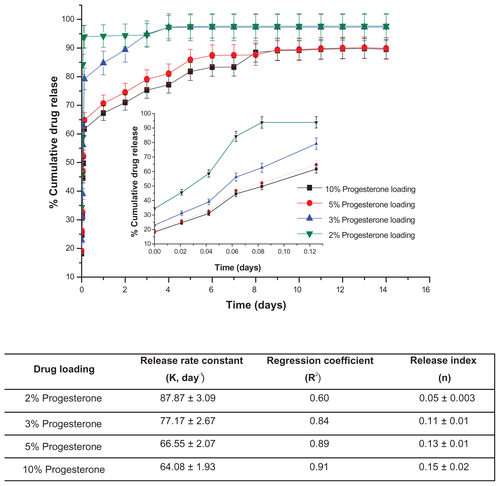
Figure S2 Effect of particle size and loading procedures on the release profile of 5-fluorouracil-loaded nanoaggregates at constant percentage of drug loading.
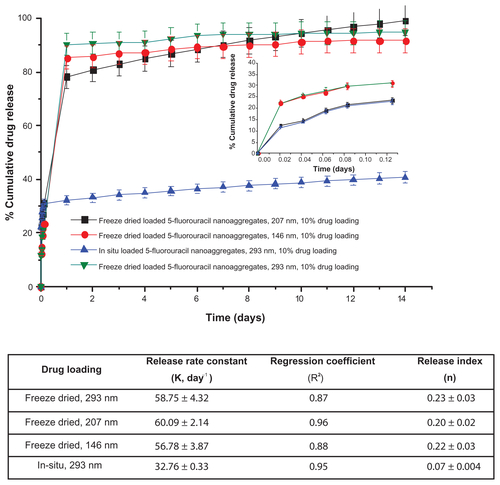
Figure S3 Modified Peppas model equation for prediction of initial burst effect of progesterone-loaded nanoaggregates prepared at 0% beta-cyclodextrin.
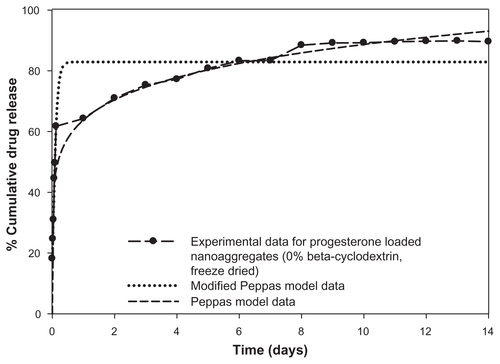
Figure S4 Modified Peppas model equation for prediction of initial burst effect of progesterone-loaded nanoaggregates sample prepared at (A) 0%; and (B) 25% beta-cyclodextrin.
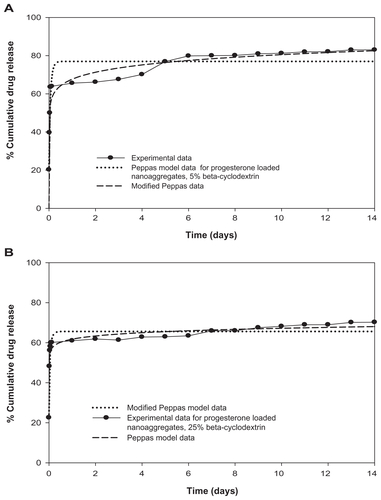
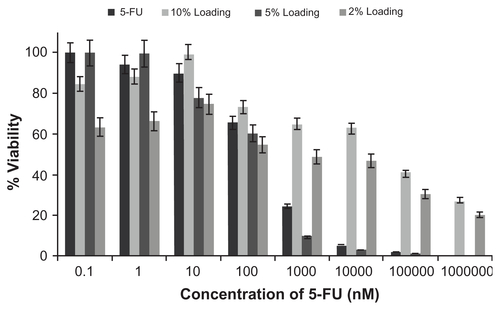
Figure S6 Effect of drug loading technique on viability of lung cancer cells.
Abbreviation: 5-FU, 5-fluorouracil.
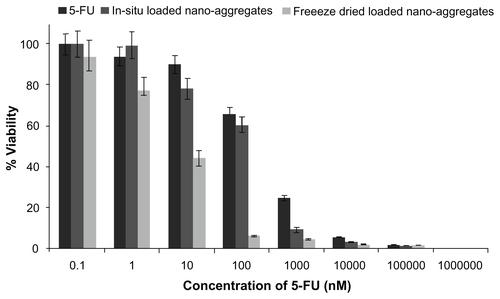
Table S1 Mathematical models describing release rates of 5-fluorouracil and progesterone from the hollow nanoaggregatesCitation14–Citation16
Table S2 Effect of drug loading on the estimated release rates and release indices according to Peppas model equation
Table S3 Effect of beta-cyclodextrin mass fraction on the release parameters of progesterone and 5-fluorouracil freeze-dried loaded samples
Table S4 Results for the curve fitting parameters of different model functions for 5-fluorouracil release profiles
Controlled release of 5-fluorouracil and progesterone from magnetic nanoaggregates.