Figures & data
Figure 1 (A) Phase contrast images of subventricular zone neural progenitor cells and their cell lineage in vitro. The subventricular zone neural progenitor cells grown in the culture media have a neurosphere form (a and b). After two weeks of culture, the subventricular zone neural progenitor cells were differentiated primarily into neurons (60%–70%) and glial cells (c and d), which were detected by staining them with a neuronal marker (TUJ1) and an astrocyte marker (glial fibrillary acidic protein) (e and f). The nucleus of the cells was counterstained with 4′,6-diamidino-2-phenylindole. Scale bars =100 μm and 200 μm. (B) The determination of ischemic lesion and transplantation of subventricular zone neural progenitor cells in the ischemic injured brain in vivo. The infarct area after middle cerebral artery occlusion injury was verified by triphenyl tetrazolium chloride staining (a) and magnetic resonance imaging (b). The subventricular zone neural progenitor cells impregnated with carbon nanotubes were transplanted into the injured brain directly by microinjection (c).
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein.
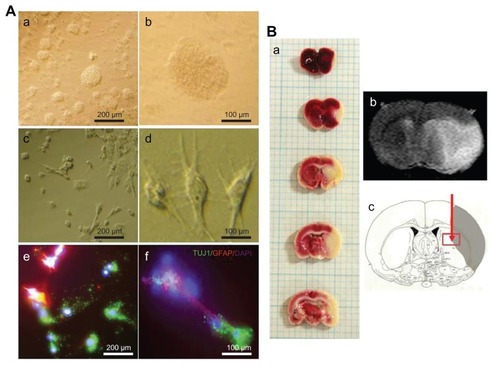
Figure 2 Quantification of infarct cyst volume and area by hematoxylin and eosin staining. Hydrophilic or hydrophobic carbon nanotubes were impregnated with subventricular zone neural progenitor cells and then transplanted into the injured brain tissue directly. Brain slices were stained with hematoxylin and eosin to (A) visualize and (B and C) measure the damaged tissue area of the brain. (B) Infarct cyst volumes and (C) infarct cyst area were measured with image-analysis software.
Notes: Scale bars =1 mm; *P < 0.05 compared to the experimental control group; #P < 0.05 compared to the subventricular zone neural progenitor cells alone group.
Abbreviations: EC, experimental control; HL + SVZ, hydrophilic carbon nanotubes impregnated with subventricular zone neural progenitor cells; HP + SVZ, hydrophobic carbon nanotubes impregnated with subventricular zone neural progenitor cells; SVZ, subventricular zone neural progenitor cells alone; W, weeks.
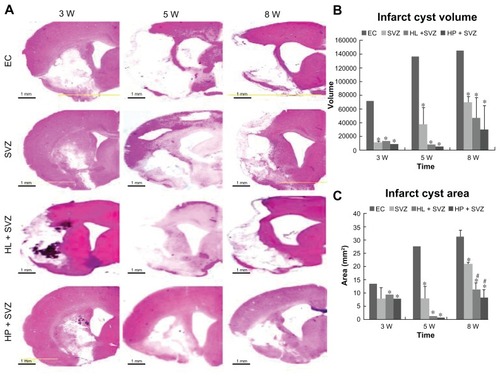
Figure 3 Analysis of behavior functional tests including (A) rotarod, (B) treadmill, and (C) vibrissae stimulated forelimb placing test after middle cerebral artery occlusion injury. Animals were subjected to injury alone, or injury-subjected animals were transplanted with subventricular zone neural progenitor cells alone, hydrophilic carbon nanotubes impregnated with subventricular zone neural progenitor cells, or hydrophobic carbon nanotubes impregnated with subventricular zone neural progenitor cells.
Notes: *P < 0.05 compared to the experimental control group.
Abbreviations: EC, experimental control; h, hours; HL + SVZ, hydrophilic carbon nanotubes impregnated with subventricular zone neural progenitor cells; HP + SVZ, hydrophobic carbon nanotubes impregnated with subventricular zone neural progenitor cells; SVZ, subventricular zone neural progenitor cells alone; W, weeks.
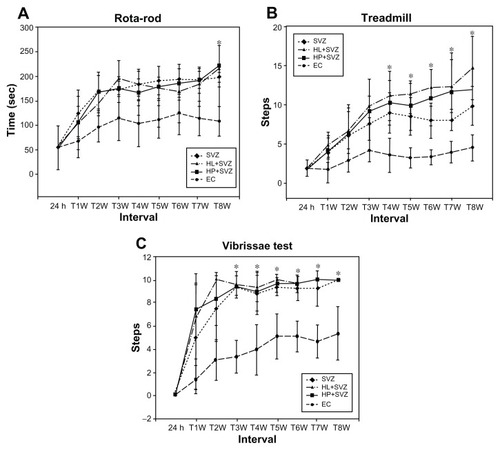
Figure 4 (A) Hydrophilic and (B) hydrophobic carbon nanotubes were impregnated with bromodeoxyuridine-stained (red; original magnification ×400) subventricular zone neural progenitor cells and transplanted onto the injury site after middle cerebral artery occlusion injury. The images show (a) the cross section of the injured brain stained with hematoxylin and eosin (where b□, c□, and d□ represent the cortex, corpus callosum, and striatum region of the brain, respectively), the migration of carbon nanotubes in the (b) cortex region, (c) corpus callosum, and (d) striatum region of the brain, and (e) diaminobenzidine staining results for nestin immunoreactivity (evidenced by brownstained cells) 1 week after transplantation.
Notes: Carbon nanotubes appear black in the histological sections and are marked by □. Arrows indicate bromodeoxyuridine-labeled subventricular zone neural progenitor cells. Original magnification for immunohistological staining was ×800. Scale bars =50 μm.
Abbreviation: CN, carbon nanotubes.
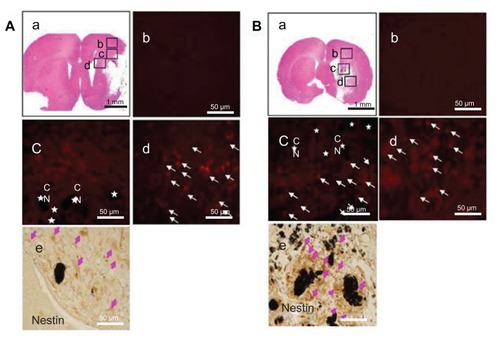
Figure 5 Doublecortin and bromodeoxyuridine staining was performed to detect the migrating exogenous subventricular zone neural progenitor cells in the hydrophilic or hydrophobic carbon nanotubes transplantation groups (A and D) 3 weeks or (E and H) 5 weeks after middle cerebral artery occlusion injury. The images show (A and E) the experimental control group (without subventricular zone neural progenitor cell transplantation), (B and F) injury-subjected rats transplanted with subventricular zone neural progenitor cells, (C and G) injury-subjected rats transplanted with hydrophilic carbon nanotubes impregnated with subventricular zone neural progenitor cells, and (D and H) injury-subjected rats transplanted with hydrophobic carbon nanotubes impregnated with subventricular zone neural progenitor cells.
Notes: Doublecortin and bromodeoxyuridine double positive immunostained cells are indicated by yellow. Scale bars =50 μm.
Abbreviations: BrdU, bromodeoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; DCX, doublecortin.
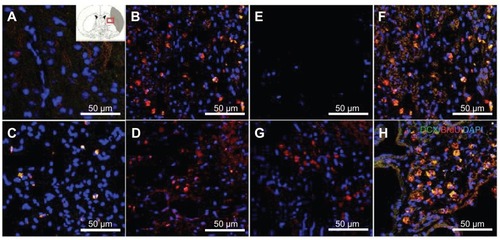
Figure 6 Images showing the neurogenesis and synapse formation based on the wettability of carbon nanotubes following middle cerebral artery occlusion injury. Microtubule-like associated protein 2 immunostaining was performed in (A) the experimental control group (without subventricular zone neural progenitor cell transplantation) and (B) the subventricular zone neural progenitor cells alone, (C) hydrophilic carbon nanotubes impregnated with subventricular zone neural progenitor cell, and (D) hydrophobic carbon nanotubes impregnated with subventricular zone neural progenitor cell transplantation groups at 5 weeks after middle cerebral artery occlusion injury. Synaptophysin (a synapse marker; red) and TUJ1 (a neuronal marker; green) – which reportedly correspond to synapses – immunostaining was carried out at 5 weeks after transplantation with (E) hydrophilic and (F) hydrophobic carbon nanotubes impregnated with subventricular zone neural progenitor cells.
Note: Scale bars =50 μm.
Abbreviation: DAPI, 4′,6-diamidino-2-phenylindole.
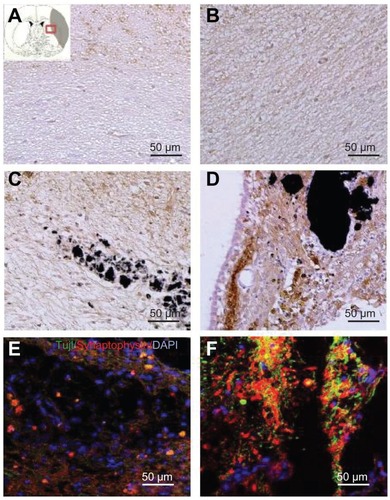
Figure 7 Immunostaining was performed with glial fibrillary acidic protein (astrocyte marker; red), and Ki67 (proliferation marker) in (A) the experimental control group (without subventricular zone neural progenitor cell transplantation) and (B) the subventricular zone neural progenitor cells alone, (C) hydrophilic carbon nanotubes impregnated with subventricular zone neural progenitor cell, and (D) hydrophobic carbon nanotubes impregnated with subventricular zone neural progenitor cell transplantation groups 5 weeks after middle cerebral artery occlusion injury. Glial fibrillary immunopositive cells are stained red and Ki67 immunopositive cells are stained green. The diaminobenzidine staining results for glial fibrillary acidic protein immunoreactivity (evidenced by brown-stained cells) in (E) hydrophilic and (F) hydrophobic carbon nanotubes impregnated with subventricular zone neural progenitor cells are also shown.
Notes: Carbon nanotubes appear black in the histological sections and are marked by □. Original magnification for immunohistological staining was ×800. Bar =50 μm.
Abbreviation: DAPI, 4′,6-diamidino-2-phenylindole.
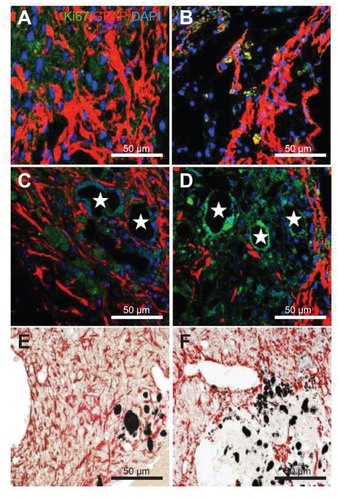
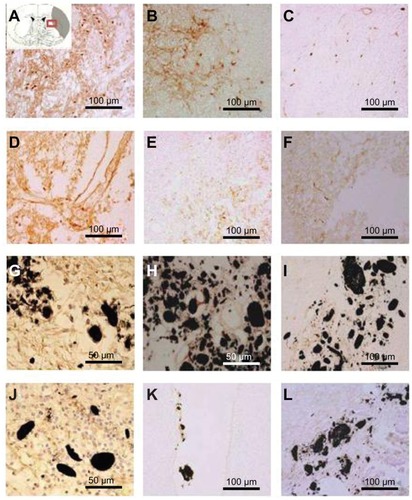
Figure 8 Immunostaining was performed with CD11b/c (OX-42) to reveal the expression of activated microglial cells (evidenced by brown-stained cells) (A, D, G, J) 1 week, (B, E, H, K) 3 weeks, and (C, F, I, L) 5 weeks after middle cerebral artery occlusion injury. The experimental groups included (A–C) the experimental control group (without subventricular zone neural progenitor cell transplantation) and the (D–F) subventricular zone neural progenitor cells alone, (G–I) hydrophilic carbon nanotubes impregnated with subventricular zone neural progenitor cell, and (J–L) hydrophobic carbon nanotubes impregnated with subventricular zone neural progenitor cell transplantation groups.
Notes: Scale bars =100 μm. Carbon nanotubes appear black.