Figures & data
Figure 1 Structure of synthetic neoglycolipids consisting of dipalmitoylphosphatidylethanolamine.
Abbreviation: DPPE, dipalmitoylphosphatidylethanolcholine; HTLV-1, human T-cell leukemia virus-1.
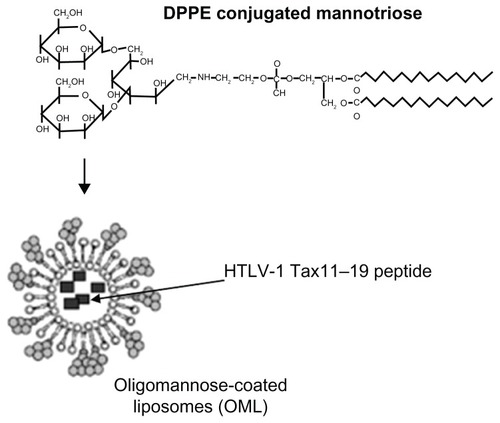
Table 1 Therapeutic efficiency of oligomannose-coated liposome-based vaccines
Figure 2 Induction of cellular immunity by intradermal immunization of mice with OML/Tax. (A) HLA-A*0201 transgenic mice (n = 5 per group) were intradermally immunized twice with OML/Tax, HTLV-1 peptide (LLFGYPVYV), or PBS on days 0 and 14. Seven days after the last immunization, the spleens and inguinal lymph nodes were collected. Inguinal lymph node cells (2 × 106/well) were stimulated in vitro with HTLV-1 peptide. Six days later, the number of IFN-γ-producing cells per 2.5, 5, or 10 × 104 inguinal lymph node cells upon stimulation with syngeneic bone marrow derived-DCs (1 × 104/well, pulsed with or without each peptide) were determined using an ELISPOT assay. IFN-γ spots were expressed as the number of peptide-loaded to peptide-unloaded target cells. *P < 0.05; **P < 0.01 vs PBS group. All experiments were performed in triplicate. Values represent the mean from five mice. Results represent the mean ± SD. (B) Freshly isolated or cryopreserved PBMCs from HTLV-1 carriers were cultured with OML/Tax, peptide alone, or without antigen. The tetramer assay was performed using fresh (ex vivo) or cultured PBMCs. The numbers in the upper right quadrants represent the percentage of tetramer+CD8+ T cells within the T lymphocyte population.
Abbreviations: DCs, dendritic cells; FITC, fluorescein isothiocyanate; IFN, interferon; HTLV-1, human T-cell leukemia virus-1; OML, oligomannose-coated liposome; PBS, phosphate-buffered saline; PE, phycoerythrin; PBMCs, peripheral blood mononuclear cells.
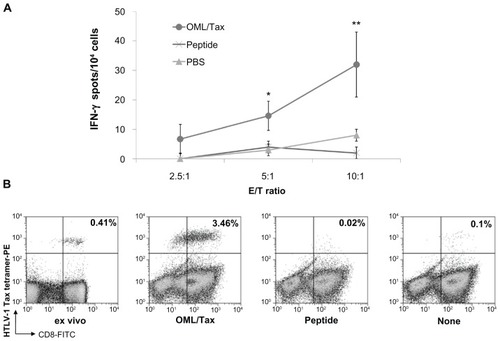
Figure 3 The induction of cellular immunity by intradermal immunization with HTLV-1 Tax/HBc chimeric particles. (A) HLA-A*0201 transgenic mice were intradermally immunized twice with HTLV-1 Tax/HBc chimeric particles (20 μg), HTLV-1 peptide (1 μg: LLFGYPVYV), HTLV-1 peptide (1 μg) plus HBc particles (20 μg) or PBS at day 0 and day 14. Seven days after the last immunization, the spleens and inguinal lymph nodes were collected. The inguinal lymph node cells (2 × 106/well) were stimulated with Tax11–19 peptide in vitro. Six days later, the frequency of cells producing IFN-γ per 5, 10, or 20 × 104 inguinal lymph node cells upon stimulation with syngenic bone marrow-derived DCs (1 × 104/well), pulsed with or without each peptide, was determined by ELISPOT assay. IFN-γ spots are expressed as the number of peptide-loaded to peptide-unloaded target cells. *P < 0.05; **P < 0.01 vs PBS group. The experiments were performed in triplicate. Results represent the mean ± SD. (B) Maturation of DCs induced by HTLV-1 Tax/HBc chimeric particles was assessed by the expression of CD86 and HLA-02 on the surface of DCs after incubation with antigens. Murine immature dendritic cells (iDCs) were obtained from bone marrow (BM) precursors. iDCs were incubated with the indicated concentrations of HTLV-1 Tax/HBc chimeric particles: 10 μg/mL of HTLV-1 Tax peptide or 10 μg/mL of phytohemagglutinin (PHA) at 37°C. Data are expressed as the mean fluorescence intensity (MFI) for each molecule compared to unpulsed (0 μg/mL) iDC controls. Results represent means ± SD of four independent experiments. *P < 0.05; **P < 0.01 vs unpulsed iDC controls.
Abbreviations: DCs, dendritic cells; IFN, interferon; HBC, hepatitis B core; PBS, phosphate-buffered saline.
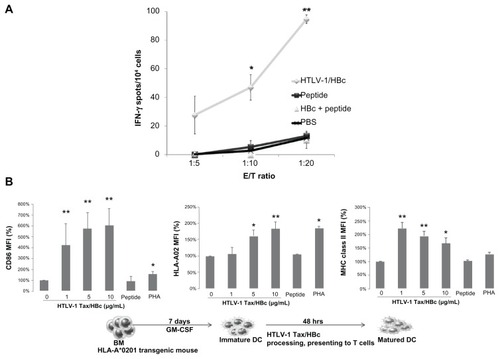
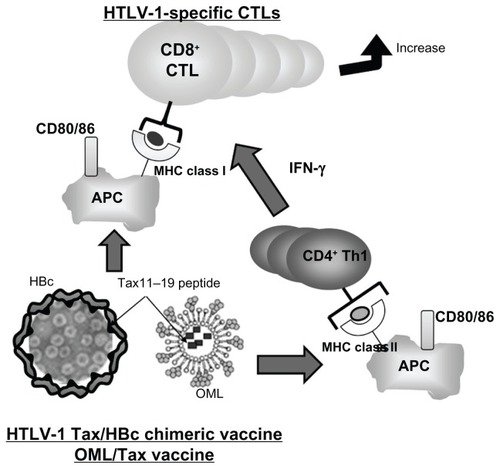
Figure 4 Strategies targeted at inducing CTLs for the prophylaxis of infectious disease and therapeutic approaches for cancer. Notes: HTLV-1 Tax/HBc chimeric particles and OML/Tax can be phagocytosed by APCs and antigenic fragments presented by either MHC class I or II molecules to activate either CD8+ T cells or CD4+ Th1 cells, respectively. Thus, this strategy could induce strong cellular immune responses without adjuvant as assessed by the increase of CD86 and MHC class I/II expression.
Abbreviations: APC, antigen-presenting cell; CTLs, cytotoxic T lymphocytes; HBC, hepatitis B core; HTLV, human T-cell leukemia virus-1; MHC, major histocompatibility complex; OMLs, oligomannose-coated liposomes; Th, T cell helper.