Figures & data
Figure 1 Various causes of neurodegeneration. Aging and inflammation due to infiltration of T lymphocytes followed by cytokines are the most common risk factors for neurodegeneration. (A) Apoptosis is considered to be programmed cell death that allows clearing out of old cells by inducing their death. However, if the same mechanism becomes dysregulated as a result of mutations, healthy cells also die, leading to neuronal loss followed by symptoms of disease. (B) Excitotoxicity is the other major cause whereby the N-methyl D-aspartate (NMDA) receptor is excessively activated by the endogenous ligand, glutamate. This drives the influx of extracellular calcium intracellularly, activating caspases which in turn destroy the nucleic acids mediating cell death. (C) Mitochondrial dysfunction due to old age or toxins generates free radicals (reactive oxygen species) that defragment DNA.
Note: These processes may occur either independently or collectively under the influence of environmental factors, medications, and infections, precipitate symptoms of neurological disorders.
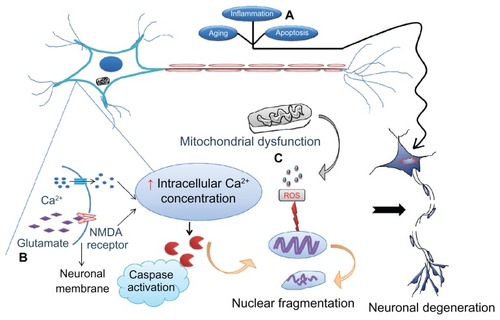
Table 1 Conventional and future therapeutics for neurological disorders
Figure 2 The tightly controlled blood–brain barrier is formed by a triad of brain capillary endothelial cells, pericytes, and astrocytes.
Notes: Surface-modified neuroprotector-loaded nanoparticles bind to brain-specific targets, eg, transferrin, lactoferrin, and low-density lipoprotein receptor-related protein receptors, and permeate into the brain, enhancing their bioavailability. Thus, the neuroprotectors released help to rejuvenate the damaged neurons.
Abbreviation: Ab, antibody; NP, nanoparticle.
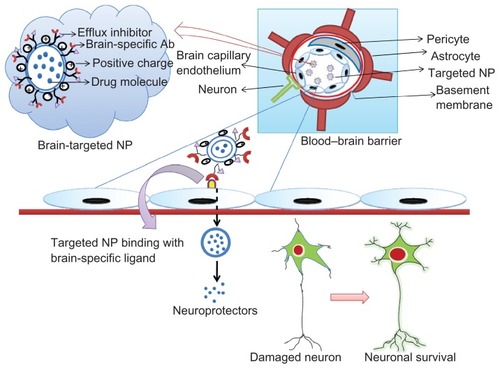
Table 2 Targeted nanotechnological applications
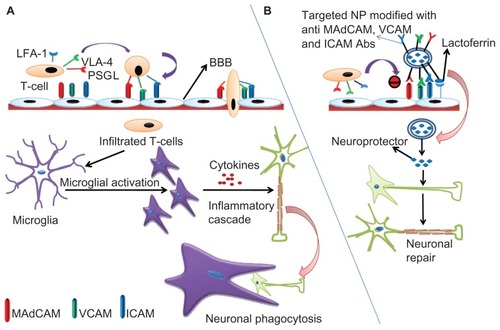
Figure 3 (A) Schematic representation of inflammatory cascade. T cells express cell surface molecules of PSGL-1, VLA-4, and LFA-1 that bind with the corresponding cell adhesion molecules, MAdCAM, VCAM, and ICAM expressed on the brain capillary endothelium and permeate into the BBB. Soon after their entry, these surface molecules activate microglial cells which, in turn, secrete cytokines driving more T cell traffic and the inflammatory cascade leading to the neuronal insult. The damaged neurons are then phagocytosed by the microglial cells. (B) Neuroprotector-loaded nanoparticles can be surface-modified by conjugation with lactoferrin and antibodies against MAdCAM, VCAM, and ICAM. Lactoferrin guides specificity for the brain due to abundant availability of low-density lipoprotein receptor-related protein (LRP) while the antibodies inhibit T cell infiltration into the BBB.
Note: Following internalization, the neuroprotector is released from the nanoparticles and initiates the repair mechanisms counteracting inflammation and its consequences.
Abbreviations: BBB, blood–brain barrier; PSGL-1, P-selectin glycoprotein ligand-1; VLA-4, very late antigen-4; LFA-1, leukocyte function-associated antigen-1; MAdCAM, mucosal addressin cell adhesion molecule; VCAM, vascular cell adhesion molecule; ICAM, intracellular adhesion molecule; NP, nanoparticle.