Figures & data
Figure 2 Gross appearances and microscopic images of postimplantation parenchyma. The number in the lower left corner of each image indicates the number of weeks following the implantation of nanofibrous membranes (NMs) or microparticles. (A) Implanted PLGA NMs degraded without causing the accumulation of transudate and exudate fluids. (B) Injected microparticles were initially dense and large (indicated by black arrows); few dense areas were observed at the end of the study. (C) Pathological examination (H&E stain) indicated no leukocyte accumulation after implantation with NMs. The number in the lower-right corner of each image indicates cell numbers (mm2). Progressively decreased cellularity was noted after chemotherapy agent loaded NMs implantation. (D) Injected microparticles (indicated by black arrows) degraded progressively and the presence of temporal inflammation reaction (accumulation of numerous inflamed leukocytes, indicated by small arrows). Magnification: 100×.
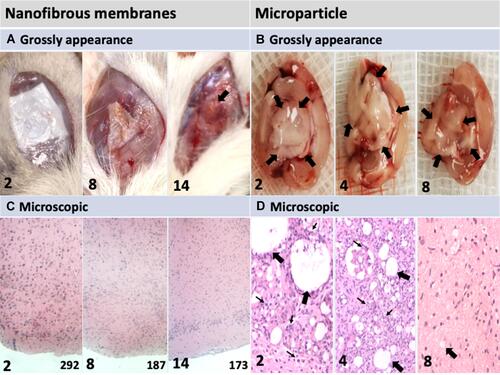
Figure 3 (A) Local delivery system that bypasses the blood–brain barrier (BBB) to reach the brain tumor. (B) NPs crossing the BBB with the aid of FUS and an external magnetic field.
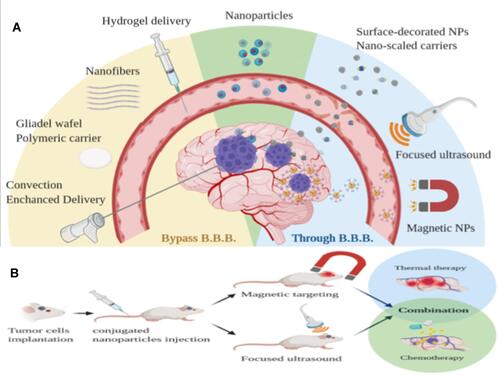
Table 1 Summary of Polymeric Vehicles for Local Delivery with Therapeutic Potential in MG Treatment
Figure 4 (A) Sustained release of a single chemotherapy agent (BCNU) from 50:50 PLGA nanofibrous membranes (NMs). (B) Sequential release of three chemotherapy agents (BCNU, irinotecan, and cisplatin) from 50:50 PLGA NMs followed by release of an antiangiogenetic agent (combrastatin) 75:25 PLGA NMs. (C) Sequential release of O6-BG from 50:50 PLGA NMs followed by release of two alkylating agents (BCNU and TMZ) from 75:25 PLGA NMs. (D) Contribution of NMs of different designs to antiglioma efficacy in an orthotopic animal model.
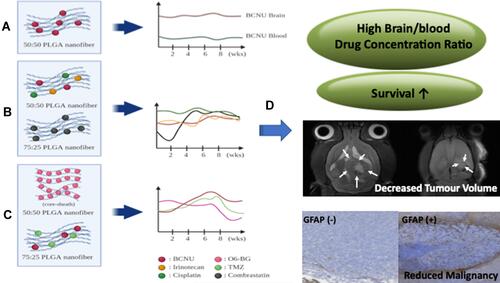
Table 2 Comparison of Advanced Local Delivery with Systemic Delivery Methods for Treating MG