Figures & data
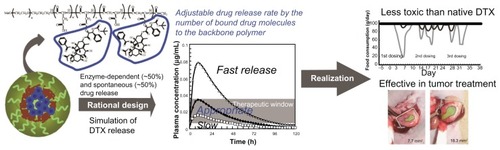
Figure 1 Determination of the optimum number of docetaxel (DTX) molecules per polymer. (A) Synthesis and chemical structure of NC-6301. Weight-average molecular weight of poly(ethylene glycol) is 10 kDa (n = 227). The number of aspartate residues (ie, w + x + y + z) was 40. The figure is a reproduction from the authors’ patent application with additional detailed information for the compound used in this study. (B) Time-dependent release of DTX to total DTX conjugated (%), with formulations of 14 (●), 12 (□), and 5 (▴) DTX molecules per polymer. (C) Plot of the values of released DTX (%) at 24 hours shown in (B) as a function of the number of DTX molecules per polymer; correlation efficient (R2) was 0.9987. (D) Scheme of the proposed release model of DTX from NC-6301 in vivo. The NC-6301 dose enters the central compartment (Vmc) via intravenous administration. NC-6301 in the central compartment (M) can be eliminated via linear (Ke) and release (Kr) pathways. The released DTX (P) in the central compartment (Vc) can distribute to the peripheral shallow (Q) and deep (R) compartments according to a three-compartment model. (E) The simulated plasma concentrations of released DTX after intravenous administration of NC-6301 based on the pharmacokinetic model, using parameters in humans. Open squares (□), closed circles (●), and open triangles (Δ) represent the time-course of released DTX when the first-order release constant of DTX from NC-6301 corresponds to 10%, 20% and 40% release in 24 hours, respectively. The dose of NC-6301 was set to be 100 mg/m2. The shaded area represents the range of concentrations required to inhibit cell growth by 50% for several cell lines.Citation18,Citation23,Citation24
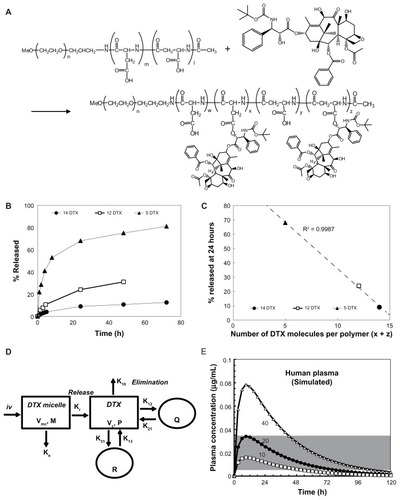
Table 1 Dose conversion between species and units: mg/kg and mg/m2
Figure 2 Properties of NC-6301 with 14 docetaxel (DTX) molecules per polymer: (A) size distribution – the average diameter was 120–130 nm, with minor disparities; (B) release of DTX as a function of time during incubation in fresh human serum (●) or sodium phosphate buffer (pH 7.4) (○) at 37°C*; (C) concentration dependency of carboxy esterase inhibitor (bis (p-nitrophenyl) phosphate sodium salt [BNPP], ▪) and protease inhibitor cocktail (PIC, ●) on the release of DTX. NC-6301 was incubated in bovine serum in the presence of each inhibitor for 6 hours at 37°C. An aliquot of the samples was taken and the released DTX concentration was determined by high-performance liquid chromatography.** (D) Actual plasma DTX level in tumor-bearing mice compared with the simulated values (dashed line for total DTX and solid line for released DTX) based on the model shown in . For actual data, plasma concentration-time profiles of total (●) and released DTX (□) after intravenous administration of NC-6301 to nude mice bearing MDA-MB-231 breast tumor at 50 mg of DTX/kg. Profiles of DTX after DTX injection at 10 mg/kg (Δ) are also shown for comparison. (E–G) Body weight changes: healthy nude mice received either DTX (E) or NC-6301 (F) intravenously three times with a 4-day interval (indicated by arrows) for determining the maximum tolerated dose.*** Body weight changes in nude mice bearing MDA-MB-231 tumor xenograft (G). The animals were also tested with regimens indicated at a 4-day interval, with seven mice per condition.****
Notes: *Each point represents the mean for three samples, and bars indicate standard deviation; **the concentration units of BNPP and PIC are mM and % (v/v), respectively, and each point represents the mean for two or three separate experiments; ***each point represents the mean for two mice; ****each point represents the mean for seven mice; bars, standard errors.
![Figure 2 Properties of NC-6301 with 14 docetaxel (DTX) molecules per polymer: (A) size distribution – the average diameter was 120–130 nm, with minor disparities; (B) release of DTX as a function of time during incubation in fresh human serum (●) or sodium phosphate buffer (pH 7.4) (○) at 37°C*; (C) concentration dependency of carboxy esterase inhibitor (bis (p-nitrophenyl) phosphate sodium salt [BNPP], ▪) and protease inhibitor cocktail (PIC, ●) on the release of DTX. NC-6301 was incubated in bovine serum in the presence of each inhibitor for 6 hours at 37°C. An aliquot of the samples was taken and the released DTX concentration was determined by high-performance liquid chromatography.** (D) Actual plasma DTX level in tumor-bearing mice compared with the simulated values (dashed line for total DTX and solid line for released DTX) based on the model shown in Figure 1D. For actual data, plasma concentration-time profiles of total (●) and released DTX (□) after intravenous administration of NC-6301 to nude mice bearing MDA-MB-231 breast tumor at 50 mg of DTX/kg. Profiles of DTX after DTX injection at 10 mg/kg (Δ) are also shown for comparison. (E–G) Body weight changes: healthy nude mice received either DTX (E) or NC-6301 (F) intravenously three times with a 4-day interval (indicated by arrows) for determining the maximum tolerated dose.*** Body weight changes in nude mice bearing MDA-MB-231 tumor xenograft (G). The animals were also tested with regimens indicated at a 4-day interval, with seven mice per condition.****Notes: *Each point represents the mean for three samples, and bars indicate standard deviation; **the concentration units of BNPP and PIC are mM and % (v/v), respectively, and each point represents the mean for two or three separate experiments; ***each point represents the mean for two mice; ****each point represents the mean for seven mice; bars, standard errors.](/cms/asset/d12230e6-b195-4e69-9f76-0948c3eae702/dijn_a_31247_f0002_c.jpg)
Table 2 Area under the concentration-time curve (AUC) values in tissues and plasma after administration of either NC-6301 at 50 mg/kg or docetaxel (DTX) at 10 mg/kg in nude mice xenografted with MDA-MB-231 human breast tumor
Figure 3 Tissue concentration-time profiles of total (●) and released docetaxel (DTX) (□) after intravenous administration of NC-6301 to nude mice bearing MDA-MB-231 breast tumor at 50 mg of DTX/kg. Profiles of DTX after DTX injection at 10 mg/kg (Δ) are also shown for comparison.
Notes: Data points indicate mean measurement for three mice; bars (standard deviation), if not shown, are within range of the symbol plots.
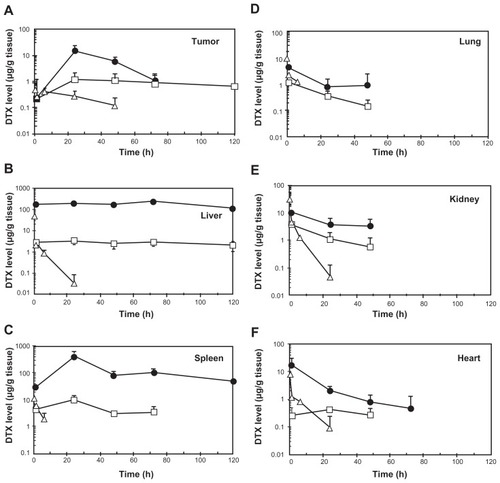
Table 3 Changes in activity of aspartate transaminase (AST) and alanine transaminase (ALT) in mice receiving intravenous NC-6301 at 50 mg/kg or intravenous docetaxel (DTX) at 10 mg/kg three times at 4-day intervals
Table 4 Changes in concentrations of creatinine (CRE) and urea nitrogen (UN) in mice receiving intravenous NC-6301 at 50 mg/kg, or intravenous docetaxel (DTX) at 10 mg/kg three times at 4-day intervals
Figure 4 Antitumor effect of NC-6301 in murine models of tumors: (A) efficacy of NC-6301 and docetaxel (DTX) against MDA-MB-231 human breast tumor. MDA-MB-231 tumor cells (3 × 106 cells/animal) were inoculated subcutaneously into the back (day −27), and intravenous administration three times at 4-day intervals (indicated by arrows) started on day 0 when tumor volumes were 218 ± 20 mm3 (average plus or minus standard error). The dose of NC-6301 is expressed as equivalent to DTX.# (B–E) Efficacy of NC-6301 and DTX against metastatic scirrhous gastric carcinoma: (B) Tumor area at the start of drug administration (day 0) – OCUM-2MLN tumor cells (5 × 106 cells/ animal) were inoculated into the subserosa of gastric walls of the mice (day −14), and intravenous administration three times at a 4-day interval started on day 0, when tumor areas were 13 ± 3 mm2 in all conditions; (C) photos of the smallest and the largest tumors on day 0 (green shading represents the tumor area); (D) tumor area of primary foci on day 14 – the doses of NC-6301 low and high represent 50 and 65 mg/kg/injection, respectively; (E) total weight of regional lymph nodes on day 14.##
Notes: #Each point represents the mean measurement for seven mice, and bars indicate the standard error; ##each point represents the individual value, each bold line represents the mean measurement for ten mice, and bars indicate the standard error; control and treated groups were compared statistically. *P < 0.05; ***P < 0.001 (Student’s t-test).
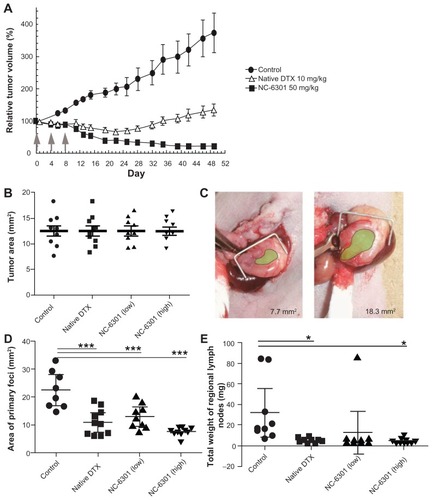
Figure 5 Toxicological tests in cynomolgus monkeys treated with intravenously administered NC-6301. Male cynomolgus monkeys received NC-6301 intravenously three times with a 2-week interval (indicated by arrows) at either 3 or 6 mg/kg. Native docetaxel (DTX) was administered similarly as a control at 3 mg/kg. (A) Average body weight change; (B) average change in food consumption; (C) changes in number of white blood cells (WBCs) and number of neutrophils.
Notes: Data points indicate mean measurements for three monkeys; bars indicate standard deviation.
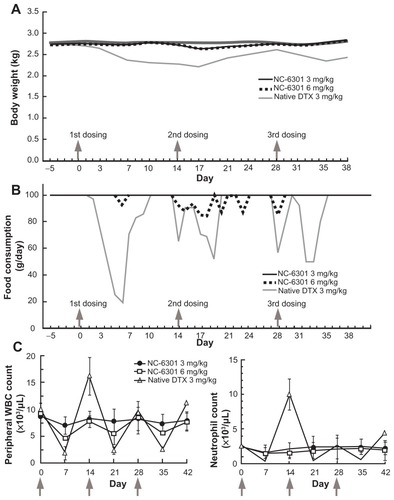
Figure 6 Graphical summary: polymeric micelle incorporating docetaxel (DTX), NC-6301, was rationally designed based on a pharmacokinetic model and proved in animal models to be effective and less toxic than native DTX.