Figures & data

Table 1 Vaccination Groups and the Formulations
Table 2 The Formulations Tested on CT-26 for IL-12 Assay and BMDCs for Maturation in vitro
Table 3 The Formulations Tested for the IL-12 Assay in vivo
Table 4 The Formulations Tested on Splenocytes and BMDCs for Cytotoxicity Assay in vitro
Figure 1 Physicochemical properties of Ng(-)pIL-12. (A) The preparation of Ng(-)pIL-12 chitosan nanoparticle. (B) Transmission electron microscopy (TEM) images of Ng(-)pIL-12. Scale bar represents 200 nm. (C) Particle size and Zeta potential of Ng(-)pIL-12. (D) Gel retardation analysis of Ng(-)pIL-12 at different nanogel/pIL-12 mass ratios as 4:1, 8:1, 16:1, respectively. (E) In vitro toxicity of Ng(-)pIL-12 and naked pIL-12 at different indicated concentrations in BMDCs and splenocytes, and the O.D. of PBS-treated group (PBS) was identified as 1. pIL-12, naked pIL-12; Ng(-)pIL-12, Ng(-) containing pIL-12. One representative from three independent experiments.
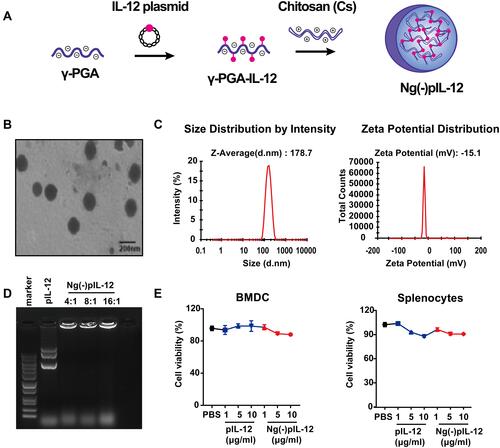
Figure 2 Ng(-)pIL-12 adjuvant induced protective effects against HBV infection quickly and successfully. (A) CT-26 cells were treated with PBS, 1 μg/mL pIL-12, Ng(-) and 9 μg/mL Ng(-)pIL-12 (with 1 μg/mL pIL-12) for 24 or 36 h, followed by further incubation with BFA (5 μg/mL) for 4 h. IL-12 expression in CT-26 cells was analysed using FACS. (B) C57 BL/6J mice were intramuscularly injected with three doses of 5 μg pIL-12 and 45 μg Ng(-)pIL-12 at two-week intervals, separately. Serum levels of IL-12 p70 were determined on day 5, 10, and 15 using ELISA after intramuscular injection. (C–F) C57 BL/6J mice were injected intramuscularly with three doses of PBS, 2 μg HBsAg alone, 5 μg pIL-12 alone, 2 μg HBsAg combined with 40 μg Ng(-) and 2 μg HBsAg combined with 45 μg Ng(-)pIL-12 at two-week intervals, separately. Anti-HBs IgG titres were measured two weeks after the second and third vaccination (C). (D–G) These immunised mice were challenged with 8 μg pAAV/HBV 1.2 on day 56 after the initiation of immunisation. Serum levels of anti-HBs IgG were detected using ELISA on day 3 and 7 after HBV challenge (D). Serum levels of anti-HBs IgG1 and IgG2b were detected using ELISA on day 10 after HBV challenge (E). Serum levels of HBsAg were detected using CLIA on day 3 after the HBV challenge (F). Intrahepatic HBV total RNA, intermediate products 3.5 kb RNA, HBV cccDNA, and HBV DNA were analysed using q-PCR and normalised to GAPDH expression on day 10 after the challenge (G). HBsAg, HBsAg alone; pIL-12, naked pIL-12; Ng(-), blank nanogels; Ng(-)pIL-12, 40 μg Ng(-) containing 5 μg pIL-12. All data represent the mean ± SEM (n ≥ 4) from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
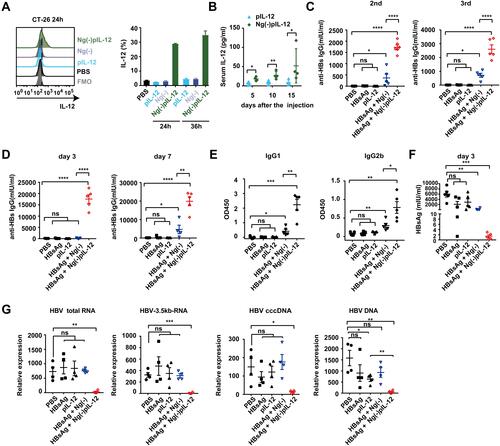
Figure 3 Ng(-)pIL-12 adjuvant induced robust HBV-specific CD8+ and CD4+ T cell responses during vaccination. C57 BL/6J mice were injected intramuscularly with three doses of PBS, 2 μg HBsAg alone, 2 μg HBsAg combined with 45 μg Ng(-)pIL-12 at two-week intervals, separately. (A) The proportion of CD11ahi CD8αlo cells among CD8+ T cells in the peripheral blood on day 7, 21 and 35 after the initiation of immunisation. (B) The proportion of CD11ahi CD49dhi cells among CD4+ T cells in the peripheral blood on day 7, 21 and 35 after the initiation of immunisation. (C) The expression of activation antigen CD69 on CD4+ CD11ahi CD49dhi cells on day 7 after the initiation of immunisation. HBsAg, HBsAg alone; Ng(-)pIL-12, 40 μg Ng(-) containing 5 μg pIL-12. All data are expressed as the mean ± SEM (n ≥ 5) from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
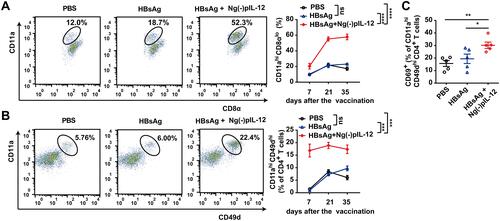
Figure 4 Ng(-)pIL-12 adjuvant promoted the maturation and antigen-presenting capacity of the CD8α+/CD103+ DCs after HBV challenge. (A) MHC-II, CD86 and CD40 expression on BMDCs incubated with PBS, pIL-12, Ng(-), Ng(-)pIL-12 for 24 or 36 h. (B–G) C57 BL/6J mice were injected intramuscularly with three doses of PBS (PBS), 2 μg HBsAg alone, 2 μg HBsAg combined with 45 μg Ng(-)pIL-12 at two-week intervals, separately, then these immunised mice were challenged with 8 μg pAAV/HBV 1.2 on day 56 after the initiation of immunisation. The proportion of CD11c+ DCs (B) and the expression of CD80 (C) on CD11c+ DCs in the peripheral blood on day 7 after the initiation of immunisation. Expression of MHC-II on CD11c+ DCs from peripheral blood on days 7, 21 and 35 after the initiation of immunisation (D). The percentage and absolute numbers of splenic CD8α+ DCs, and CD40 (MFI), CD80 (MFI) and CD86 (MFI) on CD8α+ DCs were measured on day 5 after HBV challenge (E–F). The percentage and absolute numbers of splenic CD103+ DCs, CD40 (MFI), CD80 (MFI), and CD86 (MFI) on CD103+ DCs were measured on day 5 after HBV challenge (G and H). HBsAg, HBsAg alone; pIL-12, naked pIL-12; Ng(-), blank nanogels; Ng(-)pIL-12, 40 μg Ng(-) containing 5 μg pIL-12. All data are expressed as the mean ± SEM (n ≥ 4) from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
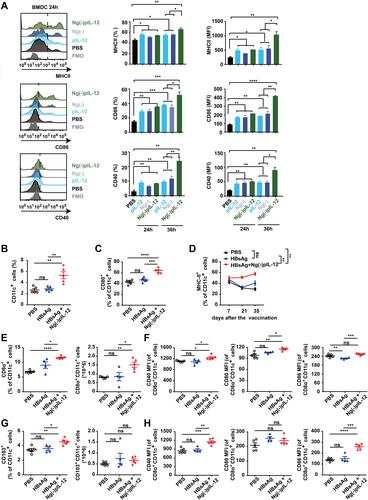
Figure 5 Ng(-)pIL-12 adjuvant induced long-lasting antiviral CD4+ T and CD8+ T cell response against HBV infection. C57 BL/6J mice were injected intramuscularly with three doses of PBS, 2 μg HBsAg alone, 2 μg HBsAg combined with 45 μg Ng(-)pIL-12 at two-week intervals, separately, then these immunised mice were challenged with 8 μg pAAV/HBV 1.2 on day 56 after the initiation of immunisation. The absolute numbers of CD8+ T cells and CD11ahi CD8αlo cells from the spleen (A) and the liver (B) on day 5 after HBV challenge. (C) LAG-3 and TIGIT expression on HBV-specific CD11ahi CD8αlo cells in the spleen on day 5 after HBV challenge. The proportion and absolute numbers of CD4+ T cells (D) and CD4+ CD11ahi CD49dhi cells (E) in the spleen on day 5 after HBV challenge. HBsAg, HBsAg alone; Ng(-)pIL-12, 40 μg Ng(-) containing 5 μg pIL-12. All data are expressed as the mean ± SEM (n ≥ 4) from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
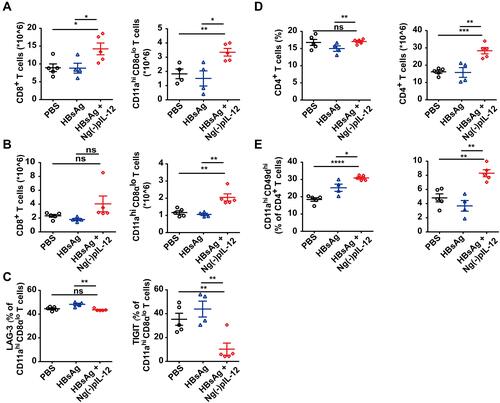
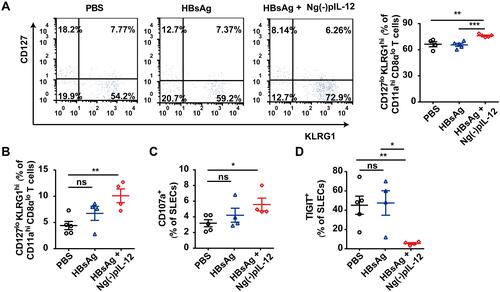
Figure 6 Ng(-)pIL-12 adjuvant triggered the terminally differentiated effector memory responses against HBV infection. C57 BL/6J mice were injected intramuscularly with three doses of PBS, 2 μg HBsAg alone, 2 μg HBsAg combined with 45 μg Ng(-)pIL-12 at two-week intervals, separately, then these immunised mice were challenged with 8 μg pAAV/HBV 1.2 on day 56 after the initiation of immunisation. (A) The percentage of SLECs among HBV-specific CD11ahi CD8αlo cells was detected on day 35 after the initiation of immunisation. (B) The percentage of splenic SLECs among HBV-specific CD11ahi CD8αlo cells on day 5 after HBV challenge. The expression of CD107a (C) and TIGIT (D) on splenic SLECs on day 5 after HBV challenge. HBsAg, HBsAg alone; Ng(-)pIL-12, 40 μg Ng(-) containing 5 μg pIL-12. All data are expressed as the mean ± SEM (n ≥ 4) from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.