Figures & data
Table 1 DL, EE, average diameter, PDI, and zeta potential of Ins-SLNs and SA-R8-Ins-SLNs
Figure 1 Transmission electron microscopic (TEM) images of SA-R8-Ins-SLNs.
Abbreviation: SA-R8-Ins-SLNs, insulin solid lipid nanoparticles modified with stearic acid–octaarginine.
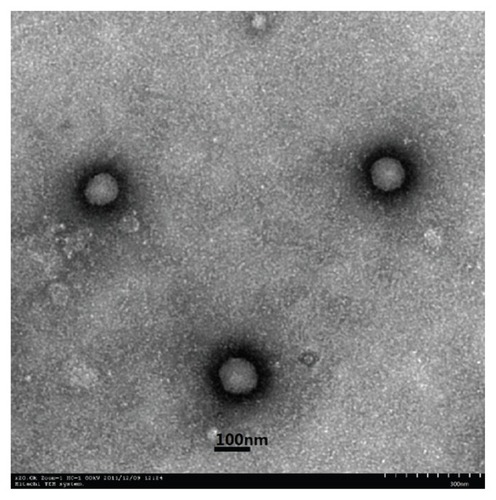
Figure 2 The remaining ratio of insulin after the incubation in SGF with pepsin.
Abbreviations: Ins-SLNs, insulin solid lipid nanoparticles; SA-R8-Ins-SLNs, insulin solid lipid nanoparticles modified with stearic acid–octaarginine; SGF, simulated gastric fluid.
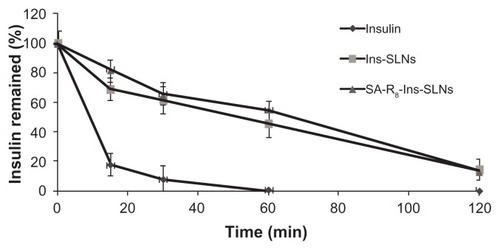
Figure 3 Remaining ratio of insulin after incubation in SIF with trypsin.
Abbreviations: Ins-SLNs, insulin solid lipid nanoparticles; SA-R8-Ins-SLNs, insulin solid lipid nanoparticles modified with stearic acid–octaarginine; SIF, simulated intestinal fluid.
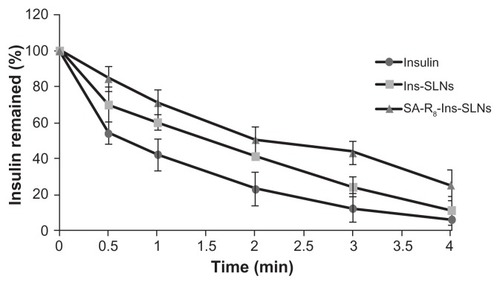
Table 2 Apparent permeability coefficient (Papp) and absorption enhancement ratio (R) for insulin across Caco-2 cell monolayers
Figure 4 Blood glucose levels changes after subcutaneous or duodenal administration to STZ-diabetic rats. (A) subcutaneous administration of insulin (2 IU/kg); (B) insulin standard solution (25 IU/kg); (C) duodenum administration of Ins-SLNs (25 IU/kg); (D) SA-R8-Ins-SLNs (25 IU/kg).
Abbreviations: Ins-SLNs, insulin solid lipid nanoparticles; SA-R8-Ins-SLNs, insulin solid lipid nanoparticles modified with stearic acid–octaarginine; STZ, streptozocin.
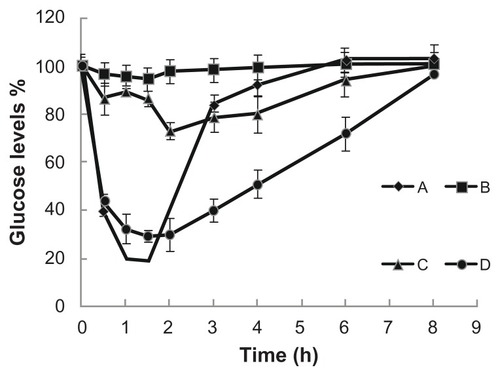
Table 3 The relative pharmacological availability of both SLNs