Figures & data
Figure 1 Structural change of nanodrugs. (A) Corresponding chemical bonding illustrations of covalent methotrexate (MTX)-carbon nano tube (CNT) and non-covalent MTX-CNT. (B) Transmission electron microscopic (TEM) images of covalent and non-covalent MTX-CNT, and the scale bar is 20 nm. (C–D) Image shows the structural differences in chemical bonds between covalently and non-covalently conjugated nanodrugs. The structures were calculated on the basis of nuclear Overhauser effect (NOE) information as described. One hundred conformers showing lowest amber energy values were obtained for each complex. The representative MTX structures with the lowest energy are shown: (C) covalent MTX-CNT and (D) non-covalent MTX-CNT. The angles between plane 1 of ring A and B and plane 2 of ring C are depicted with arrows. Several protons which showed different NOE values in both structures are depicted as well. (E) Superimposed structures between MTX attached on nanotubes (red) and PEGylated CNT (blue). The manual alignment was performed based on the orientation of the ring AB. (F) The representative distances that showed large difference between the covalent MTX-CNT (red bars) and the non-covalent MTX-CNT (blue bars) are shown in the bar chart. The distances of each pair was calculated from.Table S2
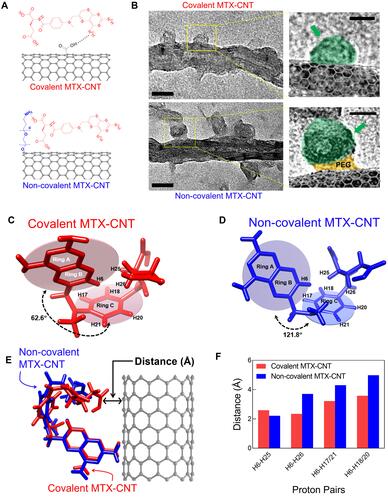
Figure 2 Physicochemical properties of nanodrugs. (A) Hydrodynamic size analysis of methotrexate (MTX), carbon nanotube (CNT), polyethylene glycolated (PEGylated) CNT, non-covalent MTX-CNT, and covalent MTX-CNT. (B) Zeta potential analysis of MTX, CNT, PEGylated CNT, non-covalent, and covalent MTX-CNT. An increase in the average size of a drug bound to CNT and a negative charge on the drug surface indicates stable drug binding. (C) Ultraviolet-visible spectra (UV-vis) of MTX, non-covalent MTX-CNT, and covalent MTX-CNT showing identical peaks at 252 nm. (D) Fourier transform infrared spectroscopy (FTIR) peaks of MTX, CNT, PEGylated CNT, non-covalent, and covalent MTX-CNT. Identical MTX peaks were observed in MTX, non-covalent, and covalent MTX-CNT.
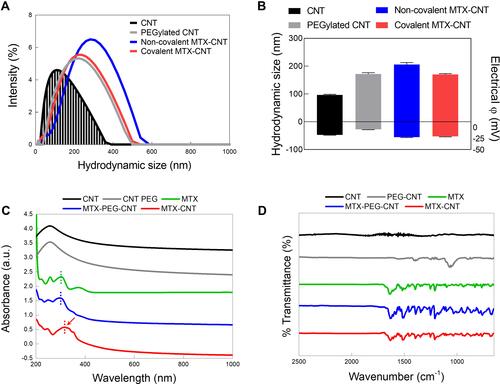
Figure 3 Drug release analysis. (A) Methotrexate (MTX) release from non-covalent MTX-carbon nanotube (CNT) and covalent MTX-CNT were analyzed in neutral condition (ie, PBS), acidic condition (ie, acetate-buffered saline (ABS)) and Fetal bovine serum (FBS) condition (ie, pH 7), (B) lysozyme-supplemented condition (ie, pH 7), and acidic lysozyme-supplemented condition (ie, pH 5) up to 288 hrs. (C) Schematic illustration of late endosome-lysosome delivery with burst drug release in the endolysosome stage. Covalently bound nanodrugs maintain stable binding compared to non-covalently bound nanodrugs in extra-cellular environments, and show burst drug release in intra-cellular environments. All data represent the mean ± SEM (n = 3). *p < 0.05, **p < 0.01 and ***p < 0.001.
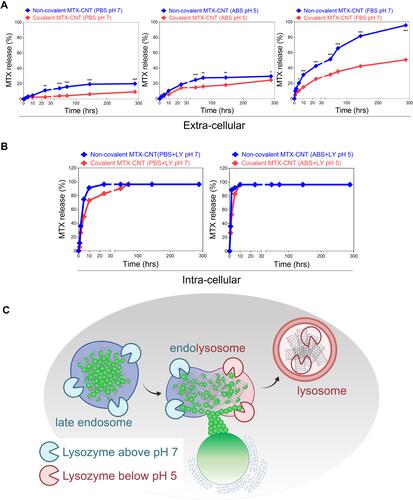
Figure 4 Human fibroblast-like synovial cell (FLS) uptake analysis. (A) Confocal images shows free FLSs and TNF-α-stimulated FLSs treated with the covalent methotrexate (MTX)-carbon nanotube (CNT) and non-covalent MTX-CNT. In FLS stimulated with TNF-a, increased uptake of nanodrugs was confirmed, and the uptake of covalent MTX-CNT was increased in FLS than that of non-covalent MTX-CNT. The scale bar is 20 μm. (B) Confocal image visualizing TNF-α-stimulated FLS treated with nano-drug. TNF-α-stimulated FLS treated with different types of absorption inhibitors were visualized and analyzed. The scale bar is 20 μm. It was shown that the uptake of covalently conjugated MTX on nanotubes FLS occurred using the caveolar-mediated endocytosis pathway, whereas the non-covalent conjugation of MTX on nanotubes was dependent on the clathrin-mediated endocytosis pathway. (C) Fluorescence intensity (uptake) analysis of free FLSs and TNF-α-stimulated FLSs treated with the nanodrug. (D) Fluorescence intensity analysis of TNF-α-stimulated FLS for comparison of major intracellular uptake pathways between covalent and non-covalently bound drugs. All data represent the mean ± SEM (n = 3). *p < 0.05 and **p < 0.01.
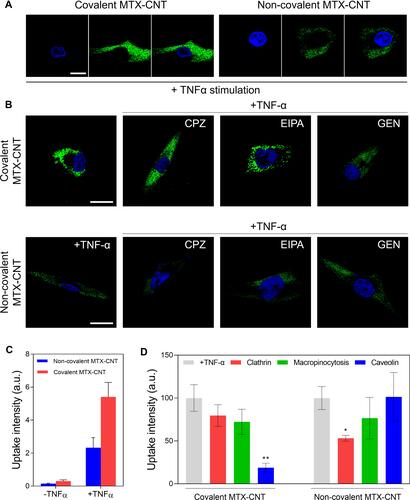
Figure 5 Intracellular trafficking. (A) Confocal microscopic analysis of early endosome (red) and covalent methotrexate (MTX)-carbon nanotube (CNT) (both covalent and non-covalent conjugation) for the desired time points (6 and 12 h). The scale bar is 20 μm. The normalized intensities of early endosomes after the treatments of covalent and non-covalent MTX-CNT at a certain time point (6 and 12 h) were quantified through calculation of fluorescence intensities of confocal images. (B) Confocal microscopic analysis of late endosome (red) and covalent MTX-CNT (green) complexes after the incubations with covalent or non-covalent MTX-CNT at indicated time points (6 and 12 h). The scale bar is 20 μm. The fluorescence intensities of late endosomes after incubating with covalent MTX-CNT and non-covalent MTX-CNT for 6 and 12 h were calculated by normalizing the fluorescence intensities of confocal images. All data represent the mean ± SEM (n = 3). ***p < 0.001.
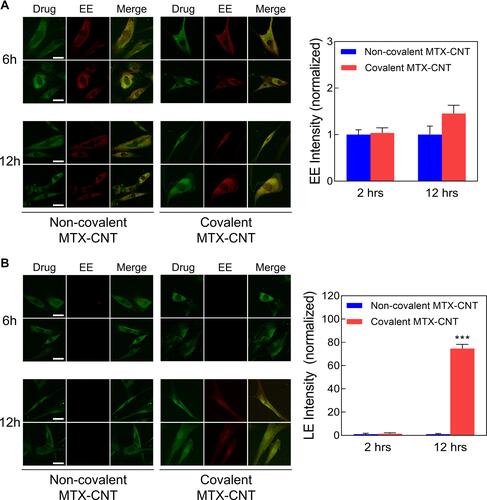
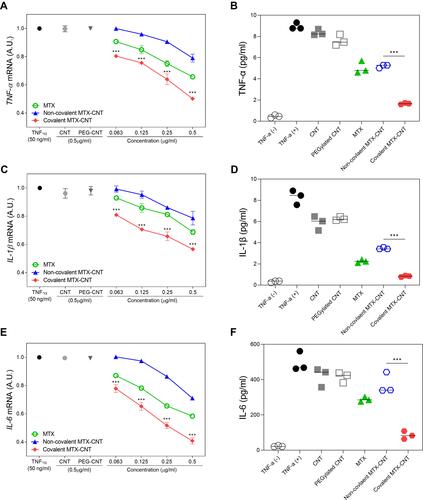
Figure 6 Anti-inflammatory efficacy. Low-dose therapeutic efficacy of covalent methotrexate (MTX)-carbon nanotube (CNT) compared to non-covalent MTX-CNT and free MTX in TNF-α-stimulated human fibroblast-like synovial cells (FLS) Suppression of (A) TNF-α, (C) IL-1-β, and (E) IL-6 mRNA levels in TNF-α-stimulated FLS. The data show that covalently conjugated MTX on nanotubes treatment led to a greater suppression of inflammatory cytokines than non-covalently conjugated MTX on nanotubes in FLSs. Suppression of protein levels was detected by ELISA in TNF-α-stimulated FLSs after treatment with nanodrugs for 24 h. (B) TNF-α, (D) IL-1-β, and (F) IL-6 protein levels were significantly suppressed by covalent MTX-CNTs compared to polyethylene glycolated (PEGylated) MTX-CNTs. All data are presented as mean ± SEM (n = 3). ***p < 0.001.