Figures & data
Figure 1 Characteristics of the antiGPC3 USPIO probes and the USPIO crystals. (A) Hitach 7600 TEM demonstrates the size and morphology of the magnetic molecular probes with a magnification of 40,000. (B) X-ray diffraction pattern of the USPIO nanoparticle assembly. The samples were deposited on glass substrates. Diffraction pattern was collected on a Shimadzu XRD-7000 diffractometer with λ = 0.15406 nm. (C) Malvern Zetasizer Nano ZS90 laser granulometer showed the mean hydrodynamic diameter of the magnetic molecular probes and their distribution. (D) Hysteresis loops of the 7 nm (red line) magnetic probes at 0.6 T.
Abbreviations: USPIO, ultrasuperparamagnetic iron oxide nanoparticle; antiGPC3 USPIO: the probe was formed using antiglypican-3 monoclonal antibodies coupled with USPIO nanoparticles.
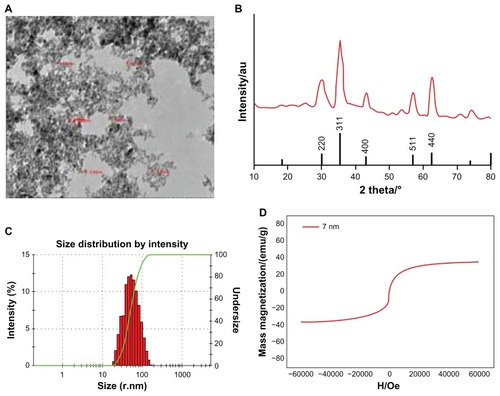
Figure 2 Hitach 7600 TEM demonstrates construction of the HPG2 cells (I–III), incubated with 750 μg/mL, 250 μg/mL, and 62.5 μg/mL iron content of antiGPC3-USPIO, antiAFP-USPIO and USPIO nanoparticles for 4 h at 37°C in 5% CO2, with a magnification of 10,000. As control, the HL-7702 hepatocytes incubated with iron content of 750 μg/mL iron content of antiGPC3-USPIO for 4 h at the same conditions were also demonstrated by TEM. The HPG2 cells took iron oxides in a concentration-dependent manner. However, the HL-7702 (IV) hepatocytes did not ingest the USPIO nanoparticles.
Note: The arrow indicates the USPIO nanoparticles.
Abbreviations: USPIO ultrasuperparamagnetic iron oxide nanoparticle; antiGPC3 USPIO: the probe was formed using antiglypican-3 monoclonal antibodies coupled with USPIO nanoparticles; antiAFP-USPIO: the probe was formed using antiAFP antibodies coupled with USPIO nanoparticles.
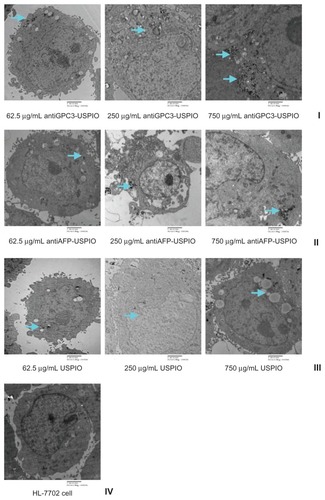
Figure 3 Hitach 7600 TEM demonstrates iron oxide incorporation by HPG2 cells (I–III) and HL-7702 hepatocytes for antiGPC3-USPIO, antiAFP-USPIO or USPIO nanoparticles in a time-dependent manner with a magnification of 10,000. The USPIO nanoparticles scattered around the cell membrane and cytoplasma after the HPG2 cells incubated with iron content of 750 μg/mL for 1 h. For 2 h and 4 h the amount of USPIO nanoparticles incorporated into intracellular increased and fused gradually, and became masses. No USPIO nanoparticle was seen in the HL-7702 hepatocytes during incubation of 1 h, 2 h and 4 h (IV).
Note: The arrow indicates the USPIO nanoparticles.
Abbreviations: USPIO, ultrasuperparamagnetic iron oxide nanoparticle; antiGPC3 USPIO: the probe was formed using antiglypican-3 monoclonal antibodies coupled with USPIO nanoparticles; antiAFP-USPIO: the probe was formed using antiAFP antibodies coupled with USPIO nanoparticles.
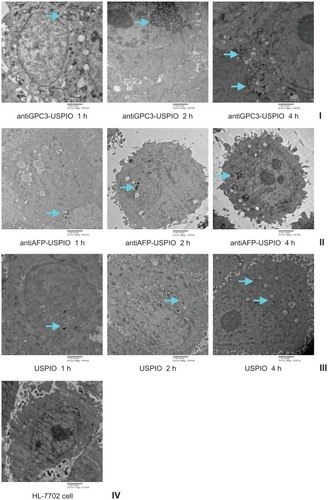
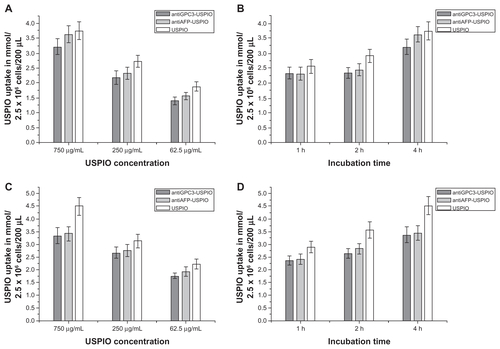
Figure 4 The iron uptakes by HPG2 cells with a concentration-dependent manner (A), by which HPG2 cells were incubated with antiGPC3-USPIO, antiAFP-USPIO and USPIO nanoparticles at iron concentrations of 62.5 μg/mL, 250 μg/mL and 750 μg/mL for 4 h under equivalent incubation conditions, or in a time-dependent manner (B), by which HPG2 cells were incubated with 750 μg/mL antiGPC3-USPIO, antiAFP-USPIO and USPIO nanoparticles for 1 h, 2 h and 4 h, respectively.
Abbreviations: USPIO, ultrasuperparamagnetic iron oxide nanoparticle; antiGPC3 USPIO: the probe was formed using antiglypican-3 monoclonal antibodies coupled with USPIO nanoparticles; antiAFP-USPIO: the probe was formed using antiAFP antibodies coupled with USPIO nanoparticles.
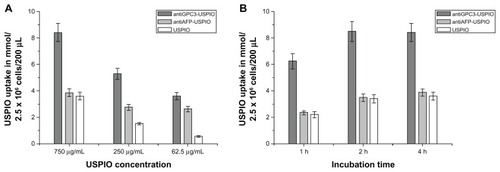
Table 1 The results of T1 and T2 values of HepG2 cells incubated with antiGPC3-USPIO, antiAFP-USPIO, and USPIO
Figure 5 The T2W images (TR/TE = 2500/62.5 ms NEX 2.0 FOV 192 × 160, slice thickness 5 mm) of tubes containing 3 mL solution of 2% agar mixed with 2.5 × 106 HepG2 cells incubated with antiGPC3-USPIO, antiAFP-USPIO and USPIO nanoparticles respectively. (A) the HepG2 cells incubated with iron content of 750 μg/mL in antiGPC3- USPIO (I), antiAFP-USPIO (II) and USPIO nanoparticles (III) for 1 h, 2 h and 4 h at 37°C in 5% CO2; the HL-7702 hepatocyte cells incubated with antiGPC3-USPIO in concentration of 750 μg Fe/mL were displayed as control (IV). (B) the HepG2 cells incubated with varied iron content (left to right: 750 μg/mL, 250 μg/mL, and 62.5 μg/mL) of antiGPC3-USPIO (I), antiAFP-USPIO (II) and USPIO nanoparticles (III) for 4 h at 37°C in 5% CO2. As control the HL-7702 hepatocyte cells incubated with antiGPC3-USPIO in corresponding iron content were also revealed (IV).
Abbreviations: USPIO, ultrasuperparamagnetic iron oxide nanoparticle; antiGPC3 USPIO: the probe was formed using antiglypican-3 monoclonal antibodies coupled with USPIO nanoparticles; antiAFP-USPIO: the probe was formed using antiAFP antibodies coupled with USPIO nanoparticles.
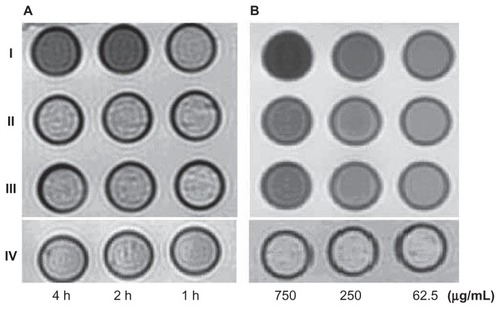
Table 2 The results of T1 and T2 values of HL-7702 hepatocyte cells incubated with antiGPC3-USPIO, antiAFP-USPIO, and USPIO
Figure S1 Identification of GPC3 protein expression in HepG2 cell. (A) Purified GPC3 protein; (B) HepG2 cell.
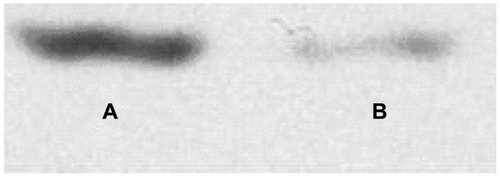
Figure S2 Hitach 7600 TEM demonstrates construction of the SMMC-7721 cells, incubated with 750 μg/mL, 250 μg/mL, and 62.5 μg/mL iron content of antiGPC3-USPIO, antiAFP-USPIO and USPIO for 4 h at 37°C in 5% CO2, with a magnification of 10,000.
Note: The SMMC-7721 cells took iron oxides increasingly in a concentration-dependent manner.
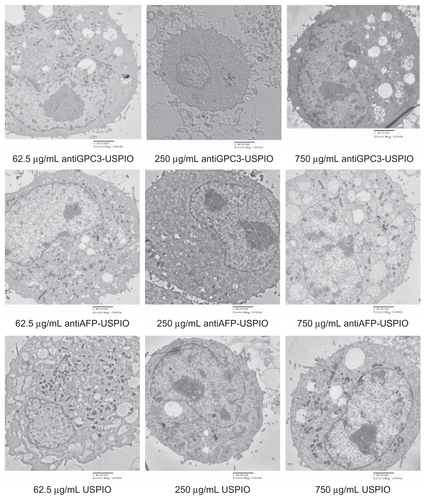
Figure S3 Hitach 7600 TEM demonstrates iron oxide incorporation by SMMC-7721 cells incubated with 750 μg/mL iron content of antiGPC3-USPIO, antiAFP-USPIO and USPIO at 37°C in 5% CO2, in time-dependent manner with a magnification of 10,000.
Notes: The USPIO nanoparticles scattered around the SMMC-7721 cell membrane and cytoplasma after 1 h incubation. For 2 h and 4 h incubation the amount of USPIO nanoparticles incorporated into intracellular increased and fused gradually, and became masses.
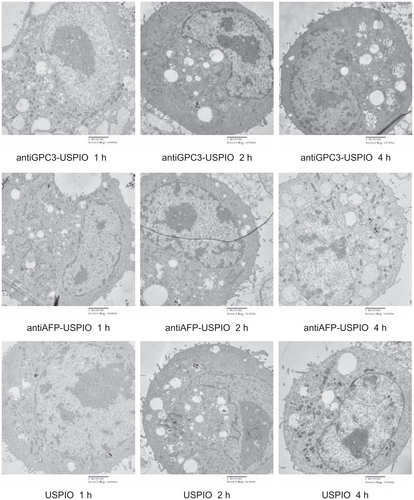
Figure S4 Hitach 7600 TEM demonstrates construction of the Hela cells, incubated with 750 μg/mL, 250 μg/mL, and 62.5 μg/mL iron content of antiGPC3-USPIO, antiAFP-USPIO, and USPIO for 4 h at 37°C in 5% CO2, with a magnification of 10,000.
Note: The Hela cells took iron oxides increasingly in a concentration-dependent manner.
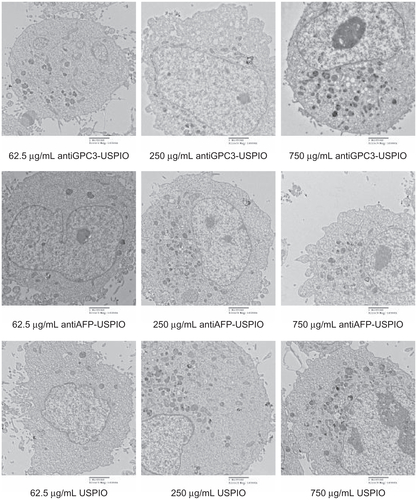
Figure S5 Hitach 7600 TEM demonstrates iron oxide incorporation by Hela cells incubated with 750 μg/mL iron content of antiGPC3-USPIO, antiAFP-USPIO, and USPIO at 37°C in 5% CO2, in time-dependent manner with a magnification of 10,000.
Notes: The USPIO nanoparticles scattered around the Hela cell membrane and cytoplasma after 1 h incubation. For 2 h and 4 h incubation the amount of USPIO nanoparticles incorporated into intracellular increased and fused gradually, and became masses.
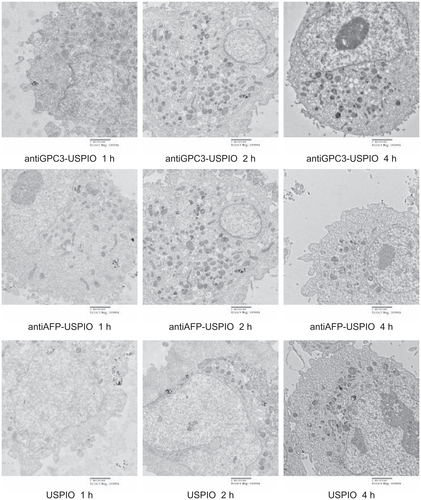
Figure S6 The T2W images (TR/TE = 2500/62.5 ms, NEX 2.0, FOV 192 × 160, slice thickness 5 mm) of tubes containing 3 mL solution of 2% agar mixed with 2.5 × 106 SMMC- 7721 cells incubated with antiGPC3-USPIO, antiAFP-USPIO, and USPIO nanoparticles respectively. (A) the SMMC-7721 cells incubated with iron content of 750 μg/mL in antiGPC3-USPIO (top row), antiAFP-USPIO (middle row), and USPIO nanoparticles (below row) for 4 h, 2 h, and 1 h at 37°C in 5% CO2; (B) the SMMC-7721 cells incubated with varied iron content (from left to right: 750 μg/mL, 250 μg/mL, and 62.5 μg/mL) of antiGPC3-USPIO (top row), antiAFP-USPIO (middle row), and USPIO nanoparticles (below row) for 4 h at 37°C in 5% CO2.
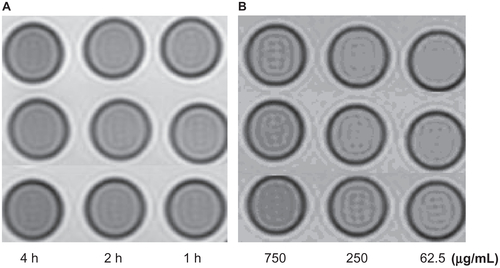
Figure S7 The T2W images (TR/TE = 2500/62.5 ms, NEX 2.0, FOV 192 × 160, slice thickness 5 mm) of tubes containing 3 mL solution of 2% agar mixed with 2.5 × 106 Hela cells incubated with antiGPC3-USPIO, antiAFP-USPIO, and USPIO nanoparticles respectively. (A) the Hela cells incubated with iron content of 750 μg/mL in antiGPC3- USPIO (top row), antiAFP-USPIO (middle row), and USPIO nanoparticles (below row) for 4 h, 2 h, and 1 h at 37°C in 5% CO2; (B) the Hela cells incubated with varied iron content (from left to right: 750 μg/mL, 250 μg/mL, and 62.5 μg/mL) of antiGPC3-USPIO (top row), antiAFP-USPIO (middle row), and USPIO nanoparticles (bottom) for 4 h at 37°C in 5% CO2.
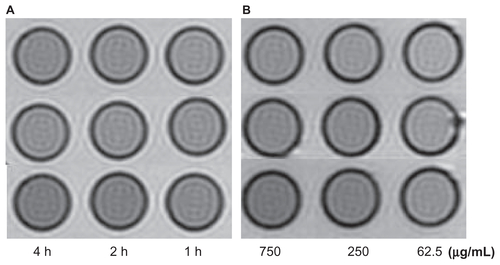
Figure S8 The iron uptakes by SMMC cells (A and B) or Hela cells (C and D) with a concentration-dependent manner, by which SMMC cells were incubated with antiGPC3-USPIO, antiAFP-USPIO, and USPIO nanoparticles at iron concentrations of 62.5 μg/mL, 250 μg/mL, and 750 μg/mL for 4 h under equivalent incubation conditions, or in a time-dependent manner, by which SMMC cells were incubated with 750 μg/mL antiGPC3-USPIO, antiAFP-USPIO, and USPIO nanoparticles for 1 h, 2 h, and 4 h, respectively.