Figures & data
Table 1 Polymerase chain reaction primers used for tryptophan hydroxylase-2 cloning and semiquantitative assay of GAPDH and tryptophan hydroxylase-2 gene expressions
Figure 1 Transmission electron microscopic image. (A) Mag-PEI and histogram showing size distribution. (B) Mag-PEI nanoparticles with high magnetic loading.
Abbreviation: Mag-PEI, magnetic poly(methyl methacrylate) core/ polyethyleneimine shell.
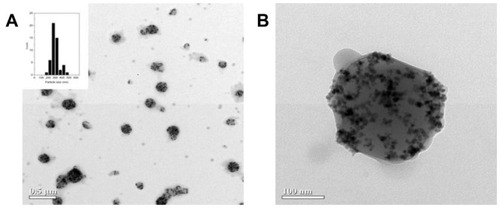
Table 2 Size and zeta potential of mag-PEI nanoparticle/DNA magnetoplex at N/P ratios of 0.4/1, 0.8/1, 1.6/1, 4.3/1, 8.7/1, and 17.5/1
Figure 2 Gel retardation assay.
Notes: One microgram of plasmid DNA was applied to the magnetoplex with mag- PEI nanoparticles at different N/P ratios. Lane 1 is the control DNA without mag-PEI nanoparticles. Lanes 2–7 represent mag-PEI NP/DNA magnetoplexes with N/P ratios of 0.4/1, 0.8/1, 1.6/1, 4.3/1, 8.7/1, and 17.5/1.
Abbreviation: Mag-PEI, magnetic poly(methyl methacrylate) core/polyethyleneimine shell.
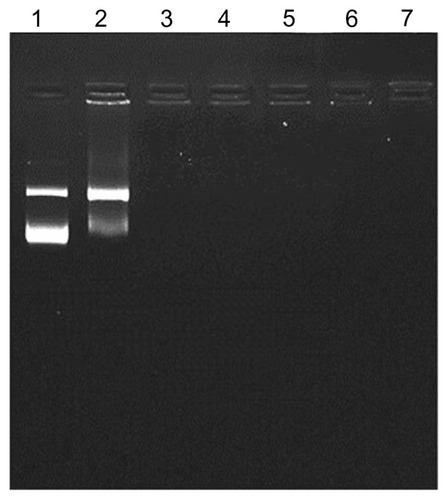
Figure 3 Atomic force microscopy images of mag-PEI nanoparticles (A) mag-PEI nanoparticles forming magnetoplexes with DNA at N/P ratios of 0.81/1 (B) and 4.3/1 (C).
Abbreviation: Mag-PEI, magnetic poly(methyl methacrylate) core/polyethyleneimine shell.
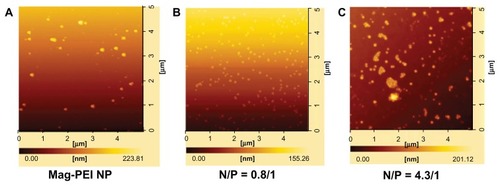
Figure 4 Transfection efficiency (A) and cytotoxicity (B) of mag-PEI nanoparticles at 15, 30, 60, 120, and 180 minutes in LAN-5 cells.
Notes: The transfection efficiency and cytotoxicity was compared with positive control Lipofectamine 2000™, PolyMAG, and negative controls (naked DNA, plasmid pGL-3-basic containing CMV promoter/enhancer). *Significant differences between cells transfected with and without a magnetic plate in each transfection reagent (P < 0.05). The gray and white bars show the results of cells incubated with or without magnetic induction, respectively.
Abbreviation: Mag-PEI, magnetic poly(methyl methacrylate) core/polyethyleneimine shell.
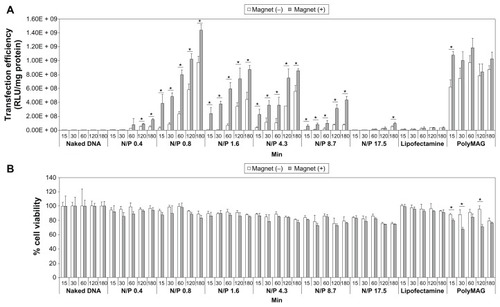
Figure 5 Confocal image of LAN-5 cells 24 hours after transfection. Cells incubated with or without a magnetic plate for (A) 60 minutes and (B) 180 minutes were used for investigation of the cellular uptake of mag-PEI nanoparticles.
Note: Green, acridine orange-stained live cells; red, RITC-stained mag-PEI nanoparticles.
Abbreviations: RITC, rhodamine-B-isothiocyanate; Mag-PEI, magnetic poly(methyl methacrylate) core/polyethyleneimine shell.
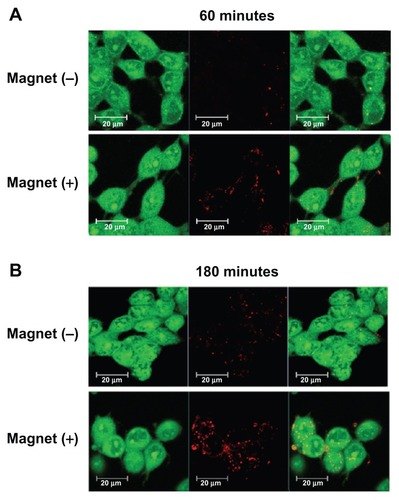
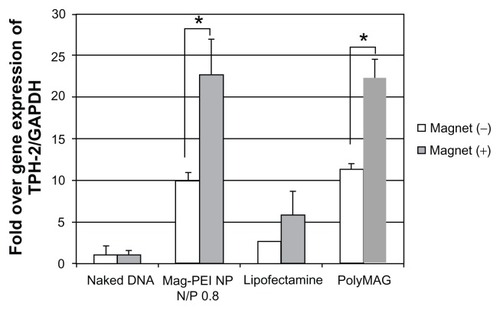
Figure 6 Semiquantitative reverse-transcriptase polymerase chain reaction result shows expression of the TPH-2 gene in LAN-5 24 hours after transfection by mag-PEI nanoparticles compared with positive control Lipofectamine 2000™ and PolyMAG, and negative control (naked DNA).
Notes: *Significant differences between cells transfected with and without magnetic plate in each transfection reagent (P < 0.05). The gray bars and white bars show the results of cells incubated with and without magnetic induction, respectively.
Abbreviations: Mag-PEI, magnetic poly(methyl methacrylate) core/polyethyleneimine shell; TPH-2, tryptophan hydroxylase-2.