Figures & data
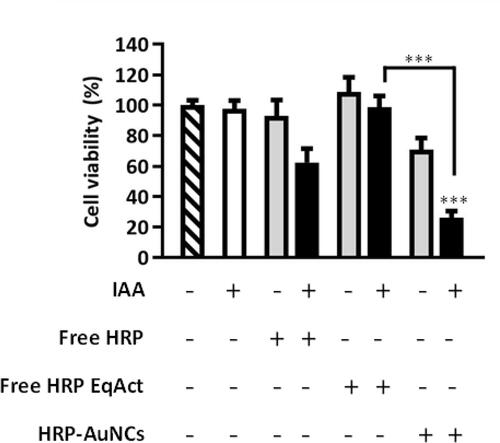
Figure 1 Representation of the design and synergistic therapeutic performance of HRP- AuNCs for EPT. A) Illustration of different steps for the assembly of HRP-AuNCs. Firstly, gold nanoparticles (AuNPs) are synthesised and functionalised with 3-MPA. Next, the carboxylic group in the gold nanoconjugates (3-MPA)-AuNCs was activated by EDC/NHS reaction. Afterwards, HRP was grafted through amide bond formation between the carboxylic group of the 3-MPA and the amine residues of the enzyme. B) Schematic representation of the enhanced therapeutic effect of HRP-AuNCs. HRP conjugated on the AuNCs leads to higher enzyme protection and internalization by endocytosis by cells (1,2). Then, the HRP oxidises the exogenous prodrug IAAleading to the production of IAA-derived free radicals and ROS (3), which induce tumour cell death by apoptosis. C) Detailed illustration of the enhanced catalytic activity provided by the enzyme on the Au s urface (3) and which produces a significant transformation of IAA into toxic radicals (4). HRP-AuNCs transforms IAA into indolyl and peroxyl radicals that lead to ROS generation and subsequent cell death by activation of apoptosis.
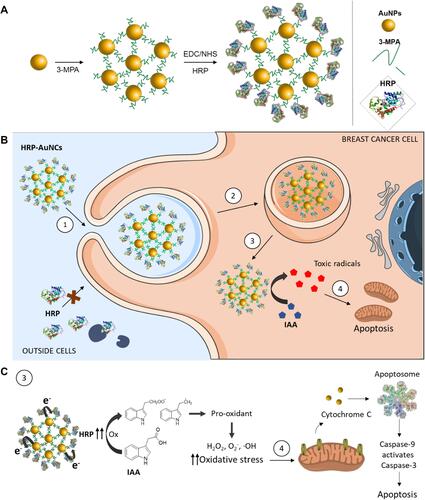
Figure 2 Characterisation of HRP-AuNCs. (A) TEM-EDX mapping of single HRP-AuNCs showing the presence of Au (from the gold scaffold), S (from the 3-MPA), and N (from the HRP). (B) UV-Vis spectra of AuNPs (red) and HRP-AuNCs (black). (C) Hydrodynamic size determined by dynamic light scattering and ζ potential of AuNPs, (3-MPA)-AuNCs and HRP-AuNCs. Data represent mean ± SD (n = 3).
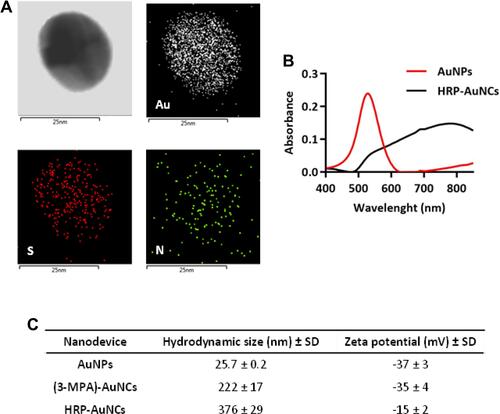
Figure 3 Biocompatibility and internalisation of HRP-AuNCs. Cytotoxicity profile of HRP-AuNCs in (A) MDA-MB-231 and (B) MCF-7 breast cancer cells. Cell viability was studied by WST-1 assay in presence of different nanoparticle dosages after 48 h of incubation. Data represent means ± SEM (n = 3). Statistically significance was determined by one-way ANOVA and Dunnett post-test (***p < 0.001). TEM images demonstrating HRP-AuNCs uptake by (C) MDA-MB-231 and (D) MCF-7 after 6 h of incubation with 50 µg/mL of nanoconjugates.
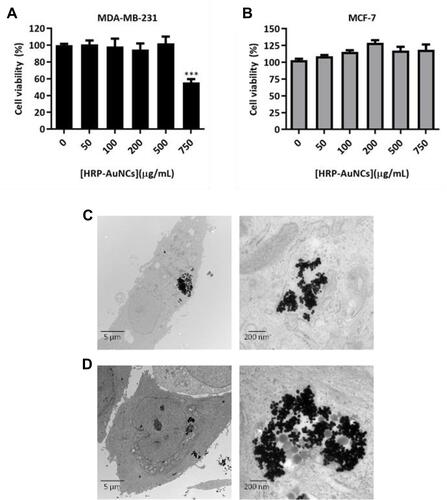
Figure 4 HRP-AuNCs for EPT in breast cancer cells. Cell viability assessment in (A) MDA-MB-231 and (B) MCF-7 treated with HRP-AuNCs at 7.85×10−2 U/mL (equivalent to 0.85 µg of HRP), free HRP EqAct at 7.85 × 10−2 U/mL (equivalent to 2.88×10−7 µg of HRP) or free HRP at 1.2 µg/mL (equivalent to 3.24 × 104 U/mL of HRP) in the absence or presence of IAA (500 µM). Cell viability was determined after 48 h of incubation by WST-1 assay. Data represent means ± SEM (n = 3). Statistically significance was determined by one-way ANOVA; Dunns post-test for MDA-MB-231 and Tuckey post-test for MCF-7 (**p < 0.025, ***p < 0.001). (C) ROS induction with the different combinations of HRP-AuNCs (7.85x10−2 U/mL, equivalent to 0.85 µg of HRP), free HRP EqAct (7.85 × 10−2 U/mL, equivalent to 2.88 × 10−7 µg of HRP) or free HRP (1.2 µg/mL, equivalent to 3.24 × 104 U/mL of HRP) in the absence or presence of IAA (IAA 500 µM) using the CM-H2DCFDA probe as an oxidative stress indicator.
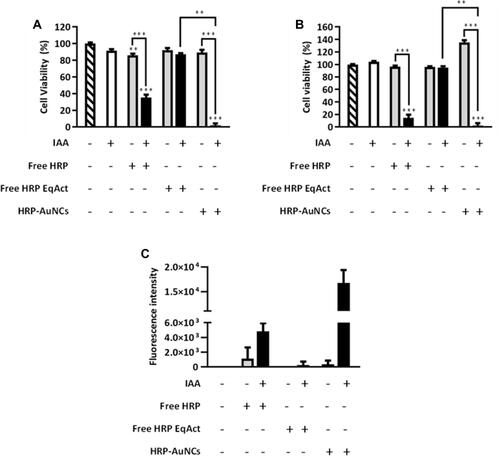
Figure 5 HRP-AuNCs for EPT in triple-negative breast cancer 3D tumour spheroid models. Cell viability assessment in MCTS formed by MDA-MB-231 cells incubated with HRP-AuNCs at 0.15 U/mL (equivalent to 1.62 µg of HRP), free HRP EqAct at 0.15 U/mL (equivalent to 5.55×10−7 µg of HRP) or free HRP at 1.2 µg/mL (equivalent to 3.24 × 104 U/mL) in the absence or presence of IAA at 250 µM. Cell viability was determined after 48 h of incubation by WST-1 assay. Data represent means ± SEM (n = 3). Significant differences were compared to control according to one-way ANOVA and Dunns post-test (***p < 0.001).