Figures & data
Figure 1 1H-NMR spectra of (A) PLGA-PEG, (B) PLGA, and (C) PEG diamine.
Abbreviations: PEG, poly(ethylene glycol); PLGA, poly(D,L-lactide-co-glycolide).
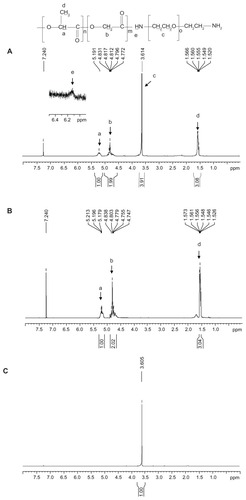
Figure 2 Transmission electron microscopic images of (A) PLGA nanoparticles and (B) PLGA-PEG nanoparticles with magnification 9600×.
Note: The insets indicated by arrows have a magnification 19,000×.
Abbreviations: PEG, poly(ethylene glycol); PLGA, poly(D,L-lactide-co-glycolide).
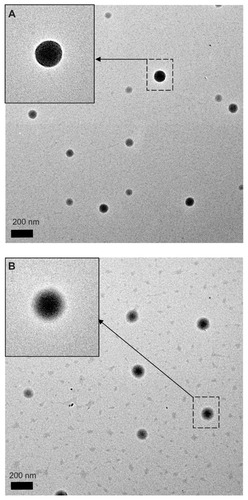
Figure 3 L929 cellular viability of (
Notes: Percentage viability of L929 cell line was analyzed by MTT assay. The values are represented as the mean ± standard deviation, n = 3. Error bars indicate the standard deviation. *P < 0.05 by Student’s t-test.
Abbreviations: PEG, poly(ethylene glycol); PLGA, poly(D,L-lactide-co-glycolide).
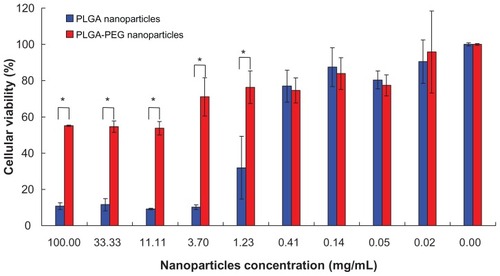
Figure 4 Effect of reaction time on (![]()
Abbreviations: PLGA, poly(D,L-lactide-co-glycolide); PEG, poly(ethylene glycol); FITC, fluorescein isothiocyanate; NP, nanoparticle.
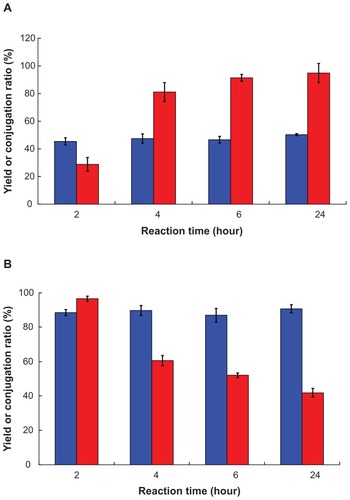
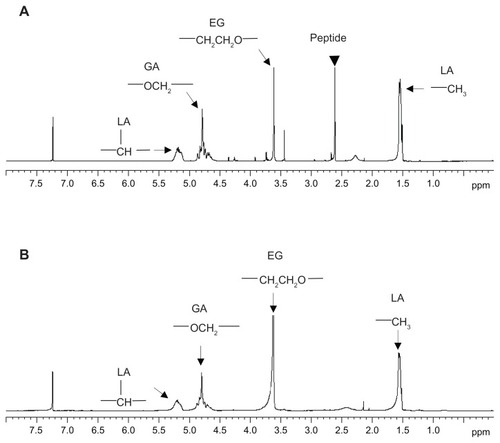
Figure 5 1H-NMR spectra of (A) peptide-conjugated and (B) peptide-free PLGA-PEG nanoparticles (400 mHz, CDCl3).
Abbreviations: PLGA, poly(D,L-lactide-co-glycolide); PEG, poly(ethylene glycol).
Abbreviations: FITC, fluorescein isothiocyanate; PLGA, poly(D,L-lactide-co-glycolide); PEG, poly(ethylene glycol).
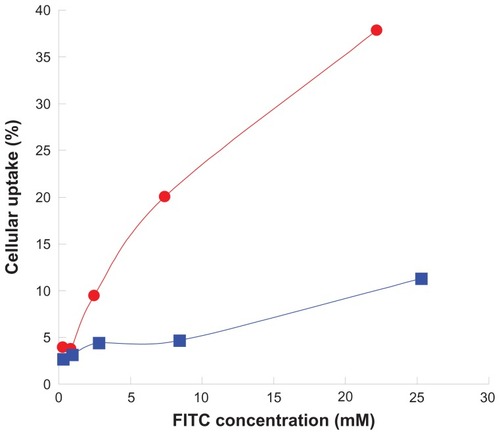
Figure 7 In vitro release of doxorubicin from peptide-free and peptide-conjugated PLGA-PEG nanoparticles in pH 4.0 (500 U lipase) and pH 7.4 release media. (
Abbreviations: PLGA, poly(D,L-lactide-co-glycolide); PEG, poly(ethylene glycol).
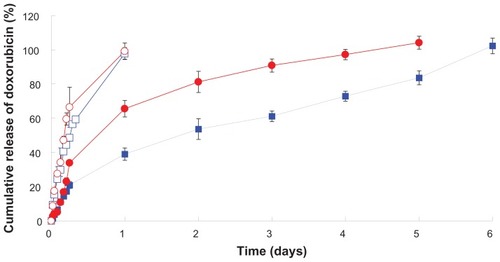
Figure 8 SKOV3 cell growth inhibition by (![]()
Abbreviations: PLGA, poly(D,L-lactide-co-glycolide); PEG, poly(ethylene glycol).
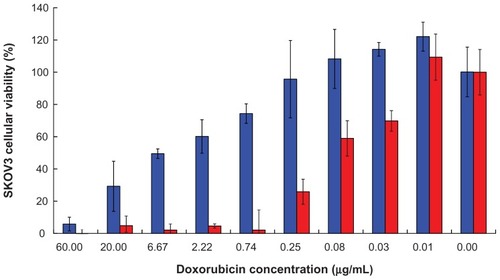
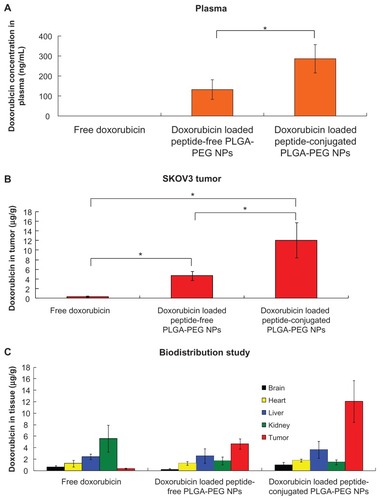
Figure 9 Accumulation and biodistribution of doxorubicin in (A) plasma, (B) tumor, and (C) tissues after intravenous administration of free doxorubicin, and doxorubicin-loaded peptide-free and peptide-conjugated PLGA-PEG nanoparticles in SKOV3 tumor-bearing mice at 24 hours.
Abbreviations: PLGA, poly(D,L-lactide-co-glycolide); PEG, poly(ethylene glycol); NPs, nanoparticles.
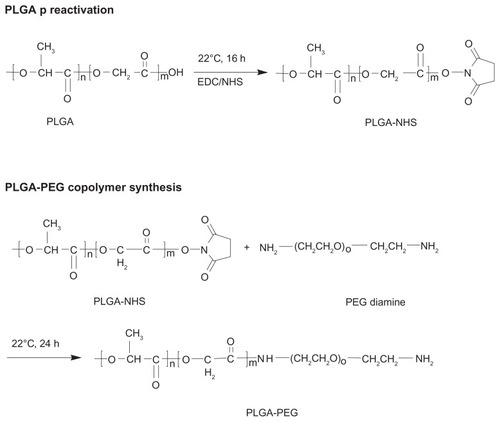
Scheme 1 Synthesis of amphiphilic PLGA-PEG copolymer bearing amino-active end group.
Abbreviation: PLGA-PEG, poly(D,L-lactide-co-glycolide)-poly(ethylene glycol).
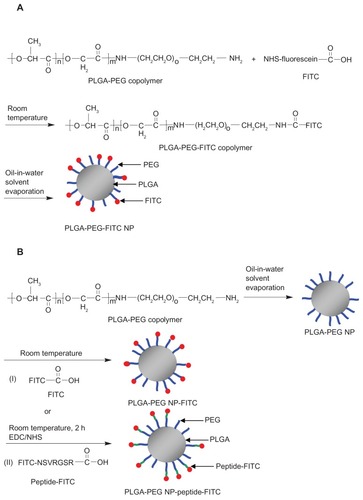
Scheme 2 Synthesis of (A) PLGA-PEG-FITC and (B) PLGA-PEG NP-FITC or PLGA-PEG NP-peptide-FITC nanoparticles.
Abbreviations: PLGA, poly(D,L-lactide-co-glycolide); PEG, poly(ethylene glycol); FITC, fluorescein isothiocyanate; NP, nanoparticle; NSVRGSR, NR7 peptide; EDC, ethyl- 3-(3-dimethylaminopropyl) carbodiimide hydrochloride; NHS, N-hydroxysuccinimide.