Figures & data
Table 1 Clinical Trials of Bacterial Tumor Therapy (https://www.clinicaltrials.gov/)
Figure 1 The illustration of fabrication of bacteria-nanoparticle bio-hybrids via chemical bonds, physical adsorption, biomineralization and other binding forms. Covalent bonds could be formed via reaction of groups on the surface of bacteria and nanoparticles; physical adsorption occurs between negative charged bacteria and positive charged nanoparticles, which are always coated with cationic polymers such as chitosan and PEI; biomineralization is the process of grabbing and turning metal ions into the element metal on the surface of bacteria; other binding forms include the bioaffinity or specific attachment such as biotin-streptavidin affinity and antigen-antibody binding.
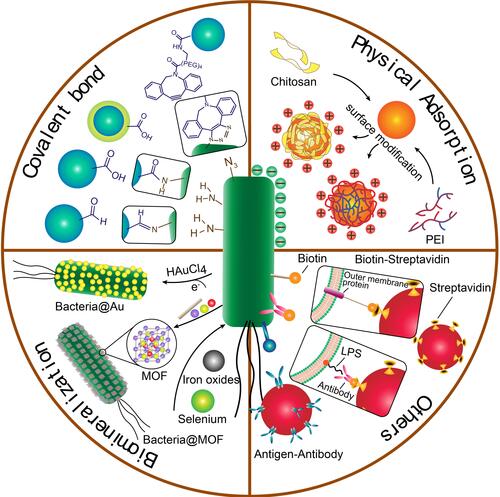
Figure 2 (A) Process of PEI employed photosensitizer nanoparticles (TDNPP)-coated live E. coli. (B) Intracellular trafficking of nanoparticle-coated live E. coli and photosensitizer delivery. Adapted with permission from Wu M, Wu W, Duan Y, Li X, Qi G, Liu B. Photosensitizer Bacteria Biohybrids Promote Photodynamic Cancer Cell Ablation and intracellular Protein Delivery. Chem Mater. 2019;31(18):7212–7220. Copyright 2019 American Chemical Society.Citation38
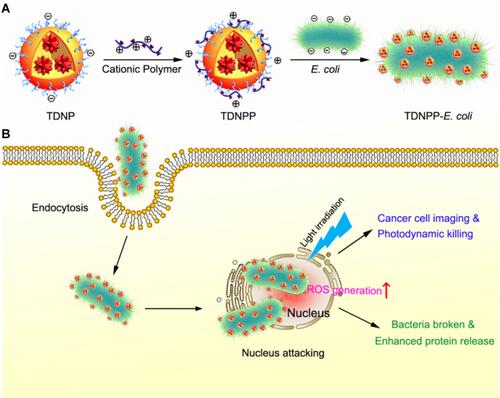
Figure 3 (A) Biomimetic mineralization of tumor-targeting E. coli by zeolitic imidazolate framework-8 (ZIF-8) for the delivery of therapeutics. (B–C) TEM images of primary E. coli and E. coli@ZIF-8/C&D. (D) Tumor growth curves of 4T1 tumor-bearing mice with different treatments (*p <0.05, **p <0.01). Adapted with permission from Yan S, Zeng X, Wang Y, Liu BF Biomineralization of Bacteria by a Metal-Organic Framework for Therapeutic Delivery Adv Healthcare Mater. 2020;9(12):e2000046. © 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.Citation47
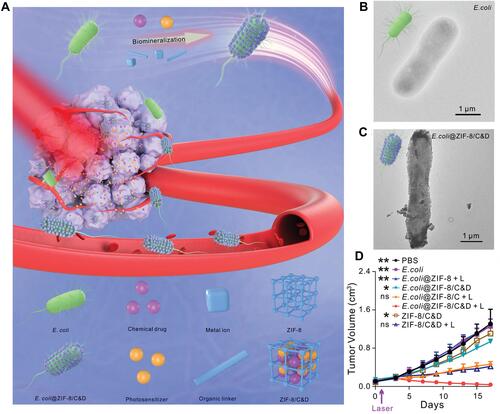
Figure 4 The development of bacteria-enabled autonomous drug delivery system (NanoBEADS) via biotin-streptavidin conjugation. (A) Each NanoBEADS agent is constructed by conjugating several streptavidin-coated PLGA nanoparticles with a tumor targeting biotinylated-antibody coated S. typhimurium VNP20009, using streptavidin–biotin noncovalent affinity-based bonds. NanoBEADS assembly was followed by incubation with mPEG-biotin to quench residual streptavidin binding sites on the nanoparticles. (B) A representative SEM image of a NanoBEADS agent. (C) Percentage occurrence of NanoBEADS formation at various nanoparticle:bacteria ratios used for NanoBEADS construction. (D) Distribution of nanoparticle loading of NanoBEADS agents constructed at nanoparticle to bacteria ratio of 100:1. (E) Distribution index (DI) of PLGA nanoparticles, NanoBEADS, and PEGylated bacteria in 4T1 tumors. Each NanoBEADS agent carries an average of 22 nanoparticles, thus, it enhances the intratumoral transport of nanoparticles by up to ≈100-fold (*p<0.05). Adapted from Suh S, Jo A, Traore MA, et al. Nanoscale Bacteria-Enabled Autonomous Drug Delivery System (NanoBEADS) enhancesintratumoral transport of nanomedicine. Adv Sci. 2019;6(3):1801309. © 2018 The Authors. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. This is an open access article distributed under the terms of the Creative Commons CC BY license.Citation54
Figure 5 The illustration of MVs engineered by genetic and non-genetic method for drug loading. Genetic manipulation was taken for reducing pathogenicity, enhancing targeting ability to tumor and improving anti-tumor immune response; non-genetic engineering modification including biomineralization, chemical bonding and membrane fusion; the functionalized MVs could load drugs such as chemotherapeutic drugs (e.g., doxorubicin, paclitaxel), nucleic acid (e.g., DNA, siRNA), photosensitizers/photothermal agents (e.g., ICG) and protein drugs (e.g., TRAIL).
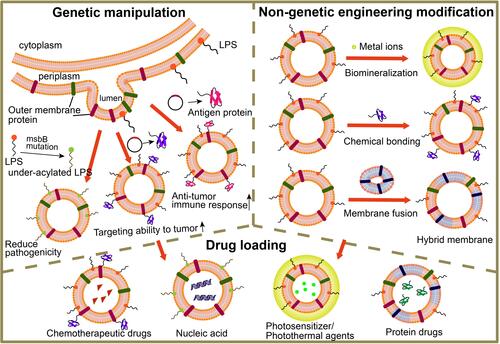
Figure 6 (A) Schematic representation of OMVs expressing HER2-specific affibody (AffiHER2OMV), purified after vesiculation from the parent bacteria and further loaded with siRNA-TAMRA (AffiHER2OMVsiRNA-TAMRA). (B) Schematic representation of the pGEX4T1-ClyA-Affibody construct. (C) Whole-body in vivo imaging revealed accumulation of AffiHER2OMVsiRNA-Cy5.5. The circles (red) indicate the tumor position. (D) Tumor growth inhibition (TGI) was monitored in HCC-1954 xenografts, and AffiHER2OMVsiRNA exerted potent antitumor effects compared with all controls (**p <0.01). Adapted with permission from Gujrati V, Kim S, Kim SH, et al. Bioengineered Bacterial Outer Membrane Vesicles as Cell-Specific Drug-Delivery Vehicles for Cancer Therapy. ACS Nano. 2014;8(2):1525–1537. Copyright 2014 American Chemical Society.Citation86
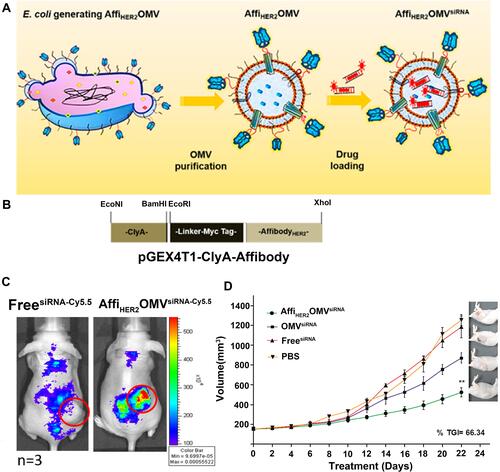
Figure 7 (A) Schematic of the membrane derived from OMV and cancer cell (CC) fusion and the resulting fused membrane camouflaged HPDA NPs to produce HPDA@ [OMV-CC] NPs. (B) Temperature elevation of HPDA NPs and HPDA@[OMV-CC] NPs (100 μg/mL). (C) CLSM image of NHDF cells, MCF-7 cells, and B16-F10 cells stained with Hoechst 33342 and cultured with DiI-dyed HPDA@[OMV-CC] NPs. Adapted with permission from Wang D, Liu C, You S, et al. Bacterial vesicle-cancer cell hybridmembrane-coated nanoparticles for tumor specific immune activationand photothermal therapy. ACS Appl Mater Interfaces. 2020;12(37):41138–41147. Copyright 2020 American Chemical Society.Citation108
![Figure 7 (A) Schematic of the membrane derived from OMV and cancer cell (CC) fusion and the resulting fused membrane camouflaged HPDA NPs to produce HPDA@ [OMV-CC] NPs. (B) Temperature elevation of HPDA NPs and HPDA@[OMV-CC] NPs (100 μg/mL). (C) CLSM image of NHDF cells, MCF-7 cells, and B16-F10 cells stained with Hoechst 33342 and cultured with DiI-dyed HPDA@[OMV-CC] NPs. Adapted with permission from Wang D, Liu C, You S, et al. Bacterial vesicle-cancer cell hybridmembrane-coated nanoparticles for tumor specific immune activationand photothermal therapy. ACS Appl Mater Interfaces. 2020;12(37):41138–41147. Copyright 2020 American Chemical Society.Citation108](/cms/asset/e5e46d9c-c9df-47da-b736-10e6a18a2a37/dijn_a_12192531_f0007_c.jpg)
