Figures & data
Figure 1 (A) Transmission electron microscopic image and (B) dynamic light scattering of silica nanoparticles coencapsulating gadolinium oxide and horseradish peroxidase.
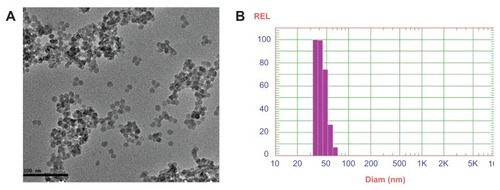
Figure 2 Energy dispersive spectroscopy of silica nanoparticles co-encapsulating gadolinium oxide and horseradish peroxidase indicating the presence of silica and gadolinium.
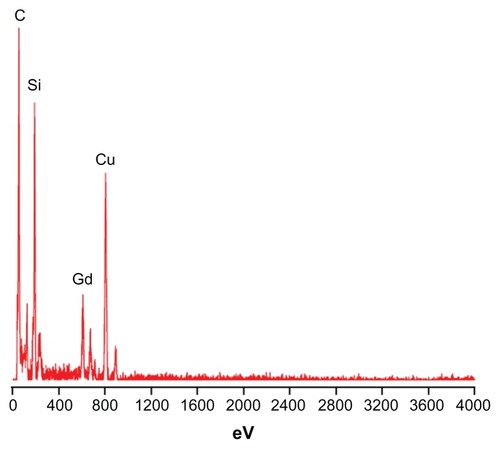
Figure 3 1H-NMR spectra of the water proton in the vicinity of silica nanoparticles (A) without gadolinium oxide and (B) with gadolinium oxide.
Abbreviation:1H-NMR, proton nuclear magnetic resonance.
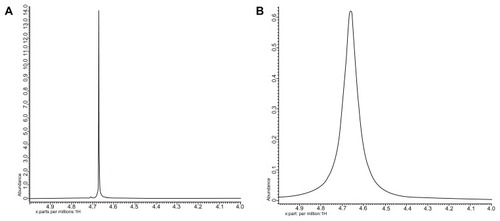
Table 1 Michaelis–Menten kinetic parameters of free horseradish peroxidase (HRP) and HRP entrapped in silica nanoparticles
Figure 4 Lineweaver–Burk plot for comparison of Michaelis–Menten parameters of free horseradish peroxidase (HRP) and HRP entrapped in silica nanoparticles.
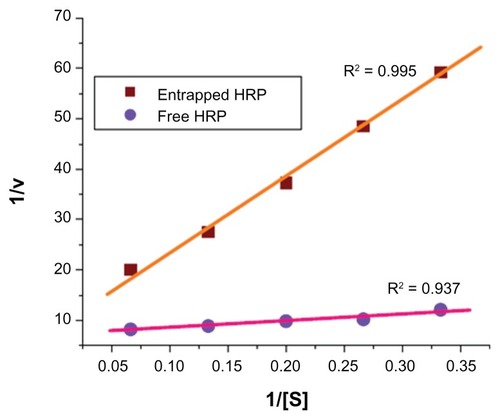
Figure 5 Temperature-dependent enzymatic activity of horseradish peroxidase (HRP; free and entrapped) during oxidation of o-dianisidine by H2O2 in phosphatebuffer (pH = 7.2) measured in the range of 20°C–70°C.
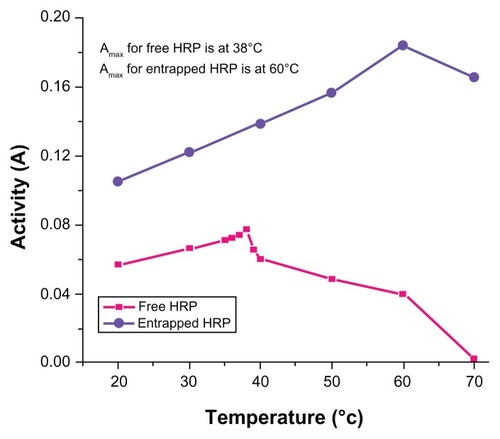
Figure 6 pH-dependent catalytic activity of horseradish peroxidase (HRP; free and entrapped) during oxidation of o-dianisidine by H2O2 in phosphate-buffer (pH = 7.2) at 25°C.
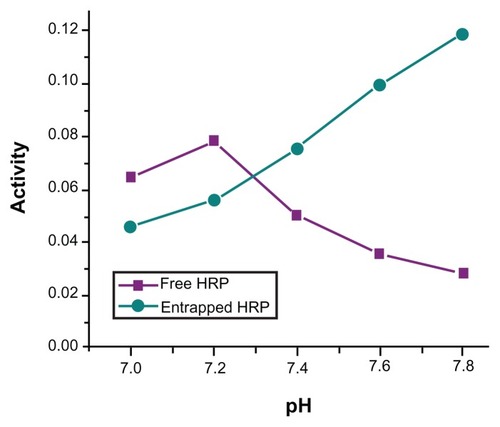
Figure 7 Kinetic study for the formation of DCF from DCFH-DA due to IAA–HRP combination.
Abbreviations: DCF, dichlorofluorescein; DCFH-DA, 2,7-dichlorofluorescein diacetate; HRP, horseradish peroxidase; IAA, indole-3-acetic acid.
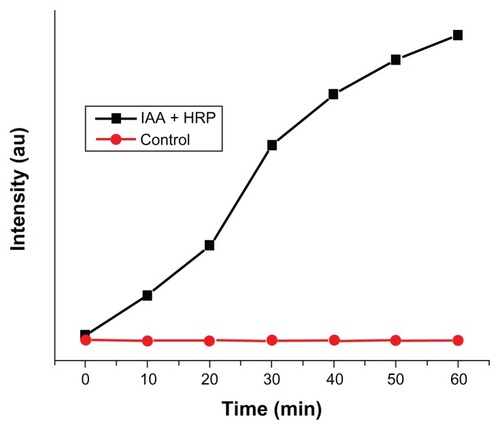
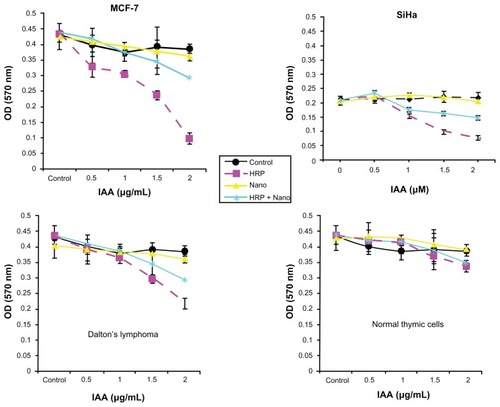
Figure 8 Cytotoxicity studies (MTT assay) of void silica nanoparticles (Nano), free HRP and HRP entrapped in silica nanoparticles (HRP + Nano) on SiHa, MCF-7, Dalton’s lymphoma, and normal thymic cell lines.
Abbreviations: IAA, indole-3-acetic acid; MTT, thiazolyl blue tetrazolium blue; HRP, horse radish peroxidase.
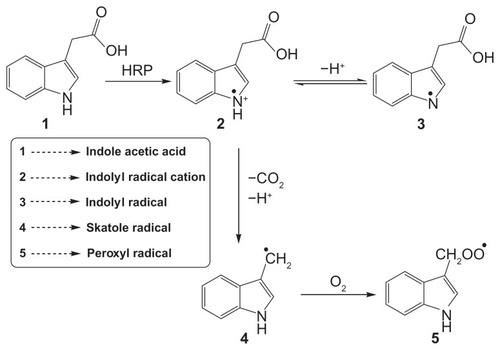
Scheme 1 Diagrammatic representation of oxidation of indole-3-acetic acid by horseradish peroxidase (HRP) to form free radicals.
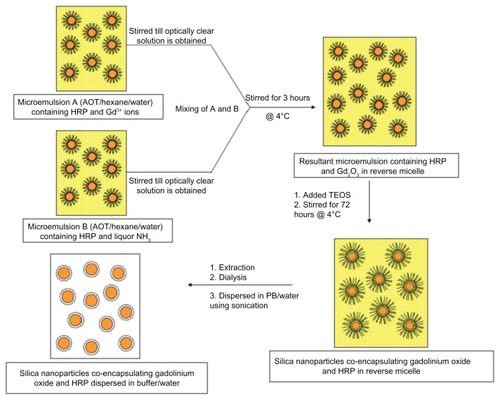
Scheme 2 Diagrammatic representation for the synthesis of silica nanoparticles coencapsulating gadolinium oxide and horseradish peroxidase using water-in-oil microemulsion.
Abbreviations: AOT, sodium bis-(2-ethylhexyl)sulfosuccinate; HRP, horseradish peroxidase; PB, phosphate-buffer; TEOS, tetraethoxysilane.