Figures & data
Table 1 Physicochemical Properties of the Liposomes
Figure 1 Determination of the antibacterial activity of SME (0−5.86 μg/mL) and/or DNS/PK (0−0.98 and 0−0.39 μg/mL) in free or liposomal form against planktonic Cutibacterium acnes during 96 h: (A) the time-killing curves of SME and enzymes in free form; and (B) the time-killing curves of SME and enzymes in liposomal form. All data are presented as the mean of three experiments±S.E.M.
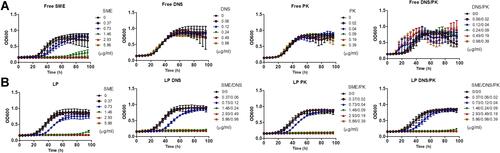
Figure 2 Determination of the antibacterial activity of SME (150 μg/mL) and/or DNS/PK (25 and 10 μg/mL) in free or liposomal form against Cutibacterium acnes biofilm after a 24-h treatment: (A) the biofilm mass determined by crystal violet staining; (B) C. acnes CFU inside the biofilm; (C) C. acnes CFU outside the biofilm; (D) PPA380 gene expression in C. acnes biofilm; and (E) PPA1035 gene expression in C. acnes biofilm. All data are presented as the mean of three experiments±S.E.M. ***p < 0.001; **p < 0.01; *p < 0.05.
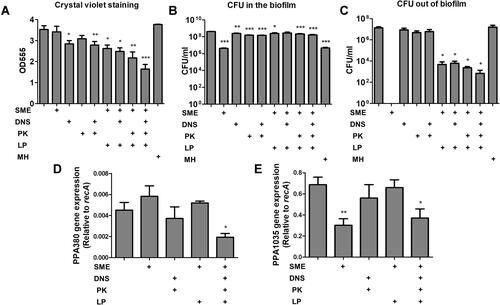
Figure 3 The antibacterial activity of SME (150 μg/mL) and/or DNS/PK (25 and 10 μg/mL) in free or liposomal form against Cutibacterium acnes biofilm determined by imaging after a 24-h treatment: (A) the biofilm structure observed by scanning electron microscopy; (B) the biofilm structure observed by confocal microscopy; (C) the biofilm thickness quantified from confocal microscopy; and (D) the biofilm mass attached on the surface of microfluidic chamber observed by confocal microscopy. All data are presented as the mean of three experiments±S.E.M. ***p < 0.001; **p < 0.01.
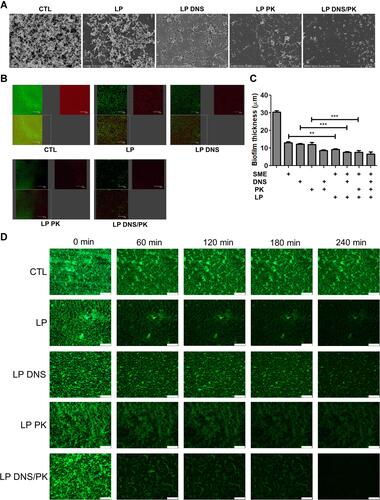
Figure 4 The penetration of liposomes containing SME (150 μg/mL) and/or DNS/PK (25 and 10 μg/mL) through Cutibacterium acnes biofilm: (A) the penetration of Bodipy FL C16-stained liposomes into the dextran-stained biofilm observed by confocal microscopy; and (B) the penetration depth of Bodipy FL C16-stained liposomes quantified from confocal microscopy. All data are presented as the mean of three experiments±S.E.M.
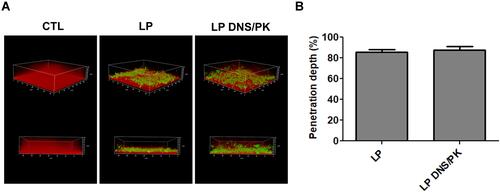
Figure 5 The penetration of liposomes containing SME (0.3%, w/v) and/or DNS/PK (0.05% and 0.02%) through pig skin in vitro after a 24-h treatment: (A) the skin deposition of rhodamine 800 in free or liposomal form; and (B) the penetration of rhodamine 800 in free or liposomal form observed by fluorescence microscopy with a vertical view. All data are presented as the mean of four experiments±S.E.M.
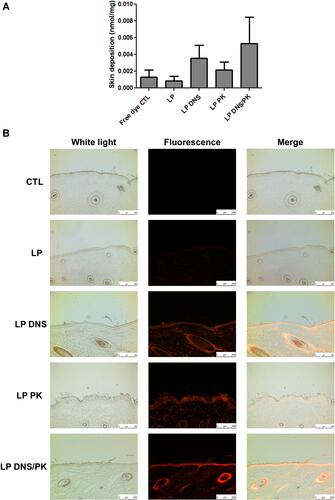
Figure 6 In vivo antibacterial activity of liposomes containing SME (0.3%, w/v) and DNS/PK (0.05% and 0.02%) with a volume of 0.2 mL against Cutibacterium acnes in skin or catheter after a 3-day treatment: (A) the C. acnes burden (labeled with XenoLight Bacterial Detection Probe 750) in skin or implanted catheter of the mice observed by in vivo imaging system; (B) the average radiance intensity of C. acnes burden in skin or implanted catheter of the mice quantified by in vivo imaging system; and (C) C. acnes CFU in skin or implanted catheter of the mice. All data are presented as the mean of six experiments±S.E.M. **p < 0.01; *p < 0.05.
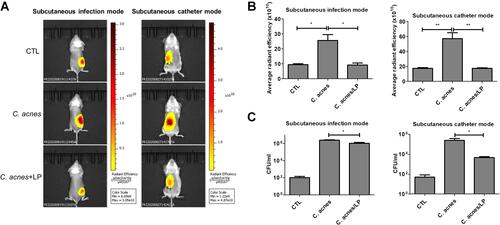
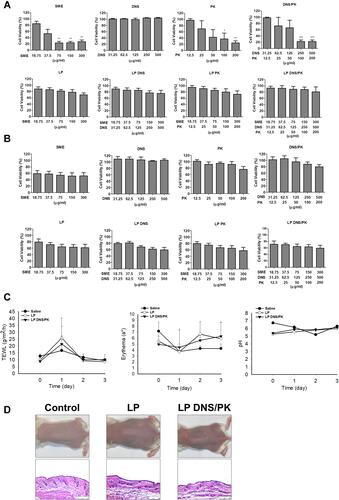
Figure 7 The safety of SME and enzymes in free or liposomal form in vitro and in vivo: (A) the cell viability of keratinocytes (HaCaT) after treatment of SME and enzymes in free or liposomal form for 24 h; (B) the cell viability of monocytes (THP-1) after treatment of SME and enzymes in free or liposomal form for 24 h; (C) the physiological parameters (TEWL, erythema, skin pH value) of the mouse skin after in vivo topical administration of liposomes; and (D) the H&E-stained histology of mouse skin treated by liposomes. All data are presented as the mean of three and six experiments±S.E.M. for cell-based and animal-based studies, respectively. ***p < 0.001; **p < 0.01; *p < 0.05.