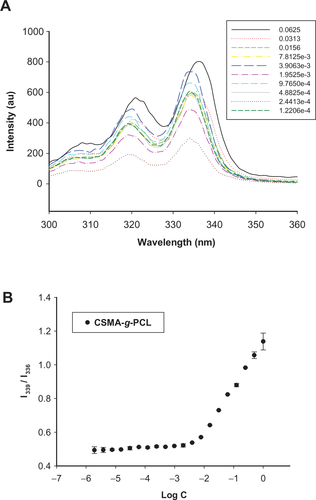
Figures & data
Figure 1 (A) FTIR spectra of PCL, CSMA, and CSMA-g-PCL. (B) 1H-NMR spectrum of CSMA-g-PCL. Peak A is attributed to two protons of PCL, Peak B to the sugar protons at the C1 position of CSMA, and Peak C to the protons on the nonreacted double bonds of CSMA. (C) TEM images of CSMA-g-PCL with (Micelle DOX) and without DOX (Micelle).
Abbreviations: FTIR, Fourier transform infrared spectrometer; NMR, Nuclear magnetic resonance spectrometry; PCL, Poly(ε-caprolactone); CSMA, Methacrylated chondroitin sulfate; CSMA-g-PCL, Poly(ε-caprolactone)-g-methacrylated chondroitin sulfate; DOX, Doxorubicin.
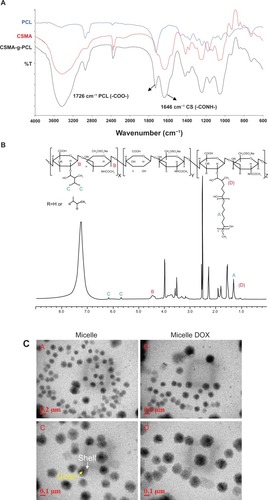
Figure 2 (A) In vitro DOX release from Micelle DOX carried out in 0.1 M PBS at pH = 6.2 or 7.4 at 37°C (n = 4). (B) Cell viability of five non-small-cell lung cancer cells exposed to Micelle DOX at various DOX concentrations. (C) Cell viability of CRL-5802 and NCI-H358 exposed to CSMA-g-PCL (n = 8).
Abbreviations: DOX, Doxorubicin; PBS, Phosphate-buffered saline; CSMA-g-PCL, Poly(ε-caprolactone)-g-methacrylated chondroitin sulfate.
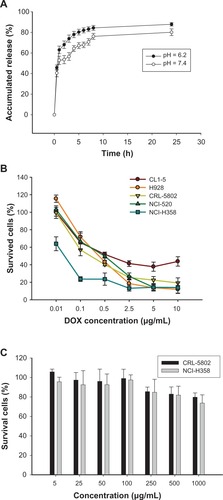
Figure 3 Flow cytometric histograms of CRL-5802 and NCI-H358-internalized DOX (blue), Lipo DOX (green), and Micelle DOX (purple) relative to the control cells at different incubation time points. Free DOX, Lipo DOX®, and Micelle DOX was tested at an equivalent DOX concentration of 0.5 μg/mL.
Abbreviations: DOX, Doxorubicin; Lipo DOX, Doxorubicin-encapsulated liposome.
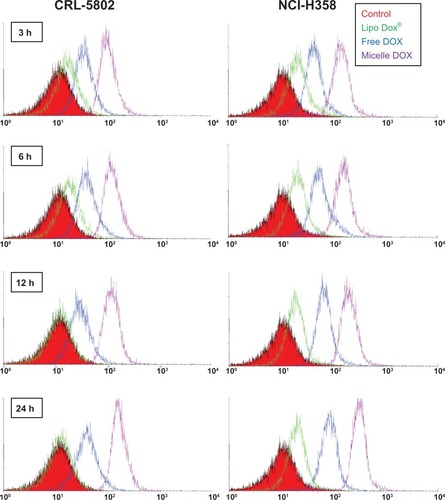
Figure 4 Confocal microscopic photographs of (A) CRL-5802, and (B) NCI-H358 internalized free DOX, and Micelle DOX at different incubation time periods (1 bar = 10 μm).
Notes: Column 1 is Micelle DOX. Column 2 is free DOX. Column 3 is the z-section image of Micelle DOX. Column 4 is the z-section image of free DOX. The red color is DOX; the blue color is DAPI-stained cell nuclei, and the purple color represents a merged image of both.
Abbreviations: DOX, Doxorubicin; DAPI, 4’,6-diamidino-2-phenylindole.
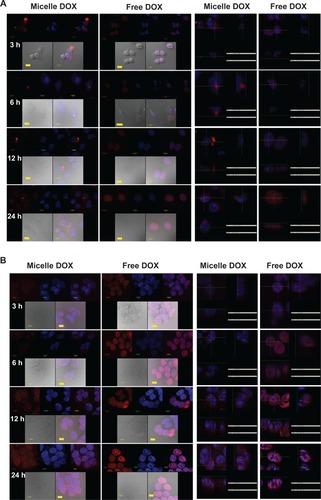
Figure 5 (A) Biodistribution in female Balb/c mice (6–8 weeks old) using a near-infrared noninvasive optical imaging technique. (B) Isolated tissues and (C) their relative fluorescent intensities after the mice were injected with IR-780-loaded CSMA-g-PCL micelle for 24 hours using an optical image system (n = 5).
Abbreviation: CSMA-g-PCL, Poly(ε-caprolactone)-g-methacrylated chondroitin sulfate.
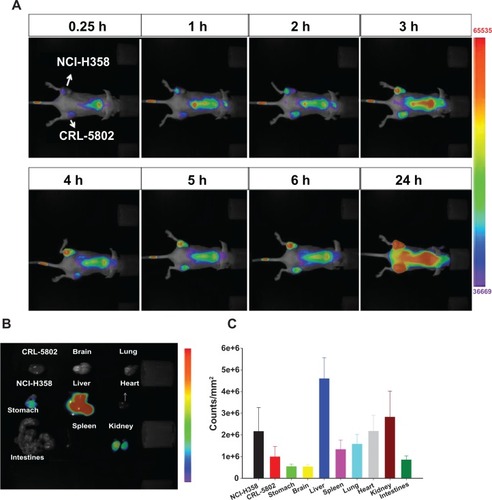
Figure 6 NCI-H-358 tumor accumulation of IR-780-loaded CSMA-g-PCL micelle in female Balb/c mice (6–8 weeks old) according to time and isolated tissues at 96 h after instillation using a NIR non-invasive optical imaging technique (n = 3 mice).
Abbreviation: CSMA-g-PCL, Poly(ε-caprolactone)-g-methacrylated chondroitin sulfate.
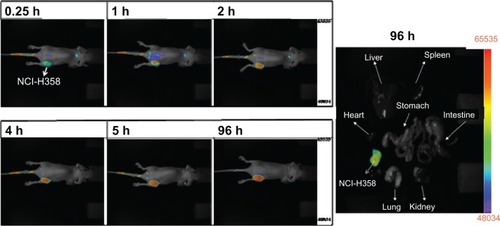
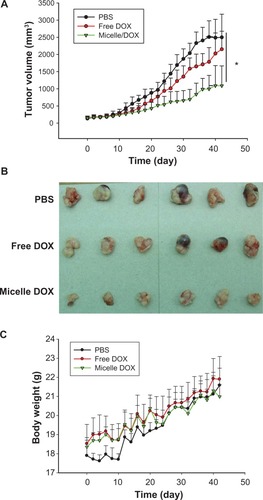
Figure 7 In vivo antitumor efficacy of free DOX and Micelle DOX in the NCI-H358 tumor-bearing model. (A) Tumor volume profiles according to time. (B) Photographic images of tumors removed at day 42 after the mice were sacrificed. (C) Body weight of the mice according to time.
Abbreviation: DOX, Doxorubicin.
Scheme 1 The chemical reaction of CSMA-g-PCL.
Abbreviations: CSMA, Methacrlyated chondroitin sulfate; MeO-PCL-OH, Methoxy-capped poly(ε-caprolactone); CSMA-g-PCL, Poly(ε-caprolactone)-g-methacrylated chondroitin sulfate; AIBN, 2,2′-azobisisobutyronitrile.
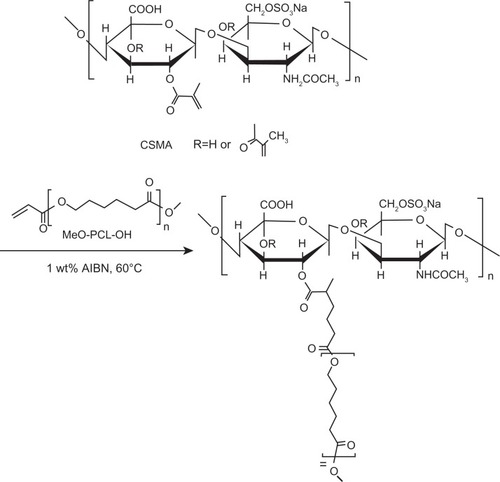
Figure S1 (A) 1H-NMR and (B) MALDI-MS of MeO-PCL-OH; (C) 1H-NMR of MeO-PCL-AC.
Abbreviations: NMR, Nuclear magnetic resonance; MALDI-MS, matrix-assisted laser desorption/ionization-mass spectrometry; MeO-PCL-OH, Methoxy-capped poly(ε-caprolactone); MeO-PCL-Ac, Methoxy-capped poly(ε-caprolactone) acrylate.
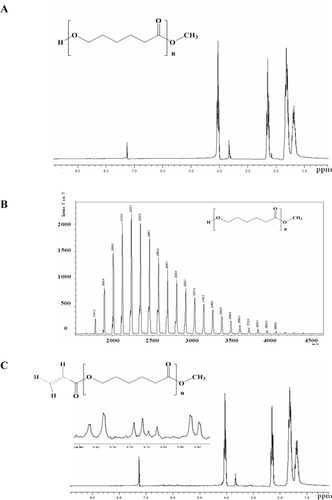
Figure S3 (A) DLS histograms and (B) zeta potentials of CSMA-g-PCL with DOX (Micelle DOX) or without DOX (Micelle).
Abbreviations: DSL, Dynamic light scattering; CSMA-g-PCL, Poly(ε-caprolactone)-g-methacrylated chondroitin sulfate; DOX, Doxorubicin.
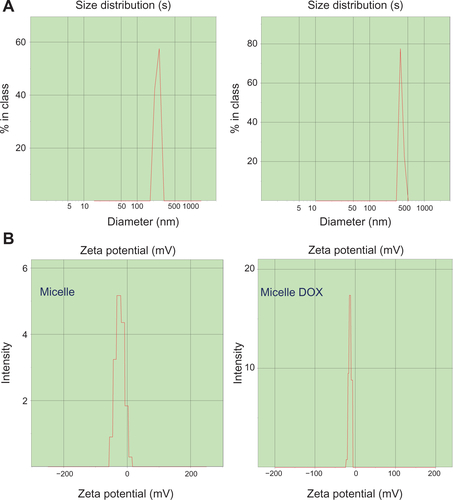
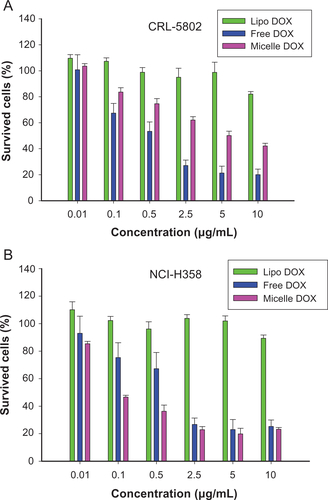
Figure S4 Cell viability of (A) CRL-5802 and (B) NCI-H358 cells exposed to free DOX, DOX-loaded liposome (Lipo DOX), and DOX-loaded CSMA-g-PCL (Micelle DOX) for 24 hours (n = 8).
Abbreviations: DOX, Doxorubicin; Lipo DOX, Doxorubicin-encapsulated liposome; CSMA-g-PCL, Poly(ε-caprolactone)-g-methacrylated chondroitin sulfate.