Figures & data
Figure 1 A schematic illustration of the medical functions and therapeutic applications of antimicrobial silver-based biomaterials in dentistry.
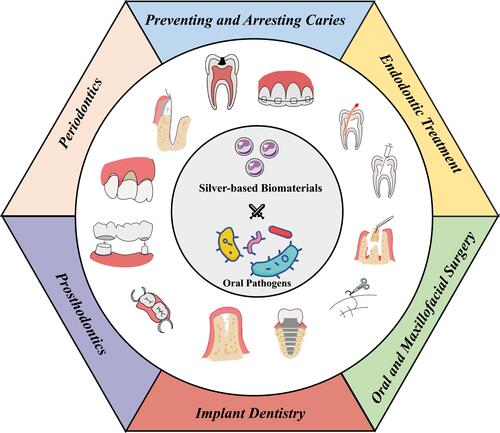
Figure 2 Antimicrobial and Dysfunction Mechanisms of AgBMs. Antimicrobial mechanisms of AgBMs (yellow frame) include destruction of the cell wall and cytoplasmic membrane, interruption of ATP production, production of destructive chemicals, interference with DNA replication and denaturation of ribosomes. The dysfunction mechanisms of AgBMs (blue frame) include efflux pump draining Ag+, flagellin aggregating AgNPs, coordinating the binding of histidine residues to Ag+, peptidoglycan membrane barrier, and biofilm barrier.
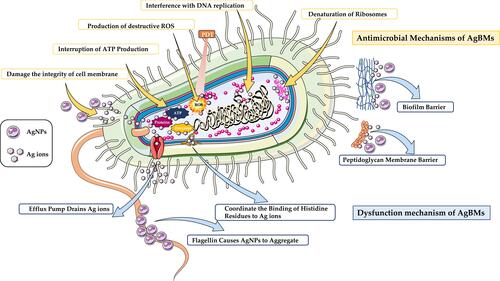
Figure 3 The AgBM was incorporated into resin composite. (A) Schematic illustration of biofilm formation by S. mutans on the surface of AgBM. (B) SEM images of the surface morphology of the AgBM specimens after immersion. (C) SEM images of the biofilm formed on the surface of the specimens. (D) CLSM representative images of the live and dead bacteria in the biofilms formed on the surface of the AgBM. *Shows the statistical significant difference . Reproduced with permission from Chatzistavrou X, Lefkelidou A, Papadopoulou L, et al. Bactericidal and Bioactive Dental Composites. Front Physiol. 2018;9:103. Copyright © 2018 Chatzistavrou, Lefkelidou, Papadopoulou, Pavlidou, Paraskevopoulos, Fenno, Flannagan, González-Cabezas, Kotsanos and Papagerakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).Citation61
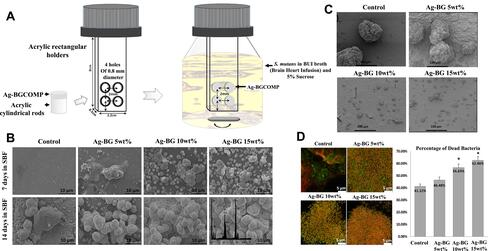
Figure 4 Orthodontic resin modified by a certain concentration of AgNPs. (A) Inhibition zones by AgNPs concentrations. (B) Colony-formation-units (Log CFU/mL) of S. mutans and L. acidophilus. (C) Tooths of the different groups exposed to microbiological caries induction. Reproduced with permission from Sánchez-Tito M, Tay LY. Antibacterial and white spot lesions preventive effect of an orthodontic resin modified with AgNPs. J Clin Exp Dent. 2021;13(7):e685–e691. © 2021 Medicina Oral S.L. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.Citation72
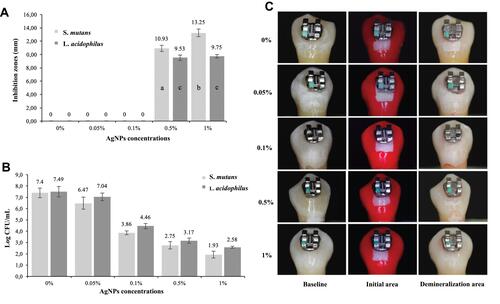
Figure 5 Application of root canal irrigation solution with AgNPs. (A) Representative images of Enterococcus faecalis biofilms after treatment with AgNPs for 5, 15 and 30 min; (B). Representative images of Enterococcus faecalis inside the dentinal tubules after treatment with irrigating solutions: AgNPs, 2% chlorhexidine or 2.5% sodium hypochlorite for 5, 15 and 30 min, respectively. Reproduced with permission from Rodrigues CT, de Andrade FB, de Vasconcelos LRSM, et al. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int Endod J. 2018;51(8):901–911. Copyright©2018 International Endodontic Journal. Published by John Wiley & Sons Ltd.Citation91
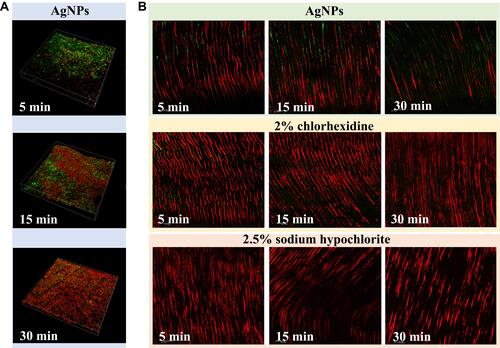
Figure 6 Application of AgBMs in Endodontic sealers. (A). Stereomicroscopic images showing apical dye penetration, and all experimental sealers showed similar sealing properties. (B). Live (green)/dead (red) images of 2-day biofilms adherent to resins. (C) Polysaccharide production by biofilms adherent to the sealer disks. (D) Colony-forming unit (CFU) counts of 2-day biofilms adherent to sealer disks. Reproduced with permission from Baras BH, Melo MAS, Sun J, et al. Novel endodontic sealer with dual strategies of dimethylaminohexadecyl methacrylate and nanoparticles of silver to inhibit root canal biofilms. Dent Mater. 2019;35(8):1117–1129. Copyright©2019 The Academy of Dental Materials. Published by Elsevier Inc.Citation107
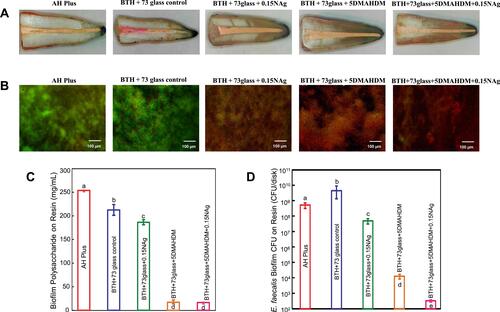
Figure 7 Applications of mesoporous silica containing AgNPs in periodontics. (A) Synthesis procedure of QAS-modified core-shell mesoporous silica containing Ag nanoparticles (Ag@QHMS). (B) The TEM images of Ag@QHMS. (C) Confocal laser scanning microscopy images of bacterial biofilms (S. aureus, E. coli, and P. gingivalis) after coculture with different nanoparticles through live/dead BacLight staining after 3 days. (D) Alkaline phosphatase (ALP) staining of BMSCs after 1 week. (E) Alizarin Red staining after 2 weeks. Reproduced with permission from Li D, Qiu Y, Zhang S, Zhang M, Chen Z, Chen J. A Multifunctional Antibacterial and Osteogenic Nanomedicine: QAS-Modified Core-Shell Mesoporous Silica Containing Ag Nanoparticles. Biomed Res Int. 2020:4567049. Copyright © 2020 Dexiong Li et al. This is an open access article distributed under the Creative Commons Attribution License.Citation115
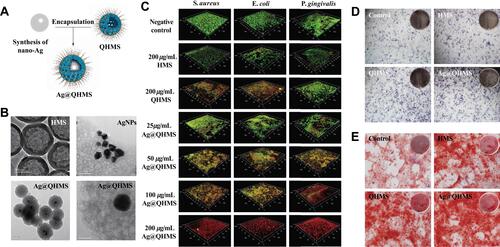
Figure 8 3D-Printable Reinforced Composite Resin-Based PMMA Modified with AgNPs. (A) Synthetic route of the nanocrystalline cellulose-silver (CNCs-Ag) composite. (B) 3D printed denture base using composite PMMA containing 1 wt% CNCs-Ag. Flexural properties and impact resistance of PMMA resin containing CNCs-Ag with different mass concentrations: (C) Flexural strength and (D) rupture work. Antibacterial activity of PMMA resin containing CNCs-Ag with different mass concentrations. (E) S. aureus; (F) E. coli. *p < 0.05 was considered statistically significant. Reproduced with permission from Chen S, Yang J, Jia YG, Lu B, Ren L. A Study of 3D-Printable Reinforced Composite Resin: PMMA Modified with Silver Nanoparticles Loaded Cellulose Nanocrystal. Materials (Basel). 2018;11(12):2444. Copyright©2018 by the Authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).Citation126
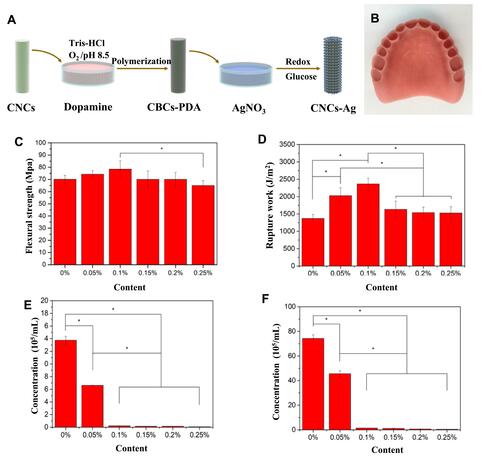
Figure 9 AgBM-modified implants promote antimicrobial property and bone formation. (A) Schematic illustration of antibacterial surface modification of Ti implants for preventing implant-associated infection. (B) Fluorescence micrographs of S. aureus and S. mutans, and (C) bacterial colony-forming units on different implant surfaces after incubation for 24 h. (A–C) Reproduced with permission from Guo C, Cu W, Wang X, et al. Poly-l-lysine/sodium alginate coating loading nanosilver for improving the antibacterial effect and inducing mineralization of dental implants. ACS Omega. 2020;5(18):10562–10571. Copyright©2020 American Chemical Society.Citation141 (D) Clinical photograph and the corresponding radiograph at 12 weeks postsurgery in labrador dogs. (E) Radiography and micro-CT evaluation of the bone tissue around dental implants. (F) Histological images demonstrating the landmarks that were used for the assessment of linear distance for the soft tissue. (G) Histological sections stained with van Gieson’s picro fuchsin. (D–G) Reproduced with permission from Qiao S, Cao H, Zhao X, et al. Ag-plasma modification enhances bone apposition around titanium dental implants: an animal study in Labrador dogs. Int J Nanomedicine. 2015;10:653–64. Copyright©2015 Dove Medical Press Limited.Citation142
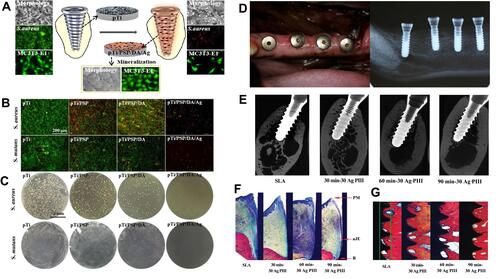
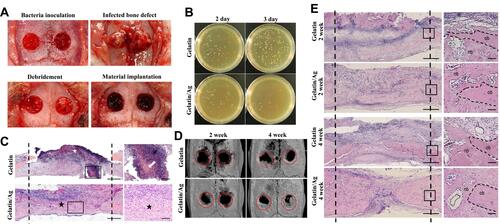
Figure 10 Gelatin sponge with colloidal silver (gelatin/Ag) promoted bone healing in infected cranial defects. (A) Creation of infected cranium defects and macroscopic appearance of the defects at main time points. (B) Antibacterial effect of Gelatin/Ag after debridement. (C) Representative images of HE-stained sections at 2 days after debridement. (D) Three-dimensional reconstructions of the cranium at 2 and 4 weeks. (E) Representative images of HE-stained sections of defects at 2 and 4 weeks. Reproduced with permission from Dong Y, Liu W, Lei Y, et al. Effect of gelatin sponge with colloidal silver on bone healing in infected cranial defects. Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 1):371–377. Copyright©2016 Published by Elsevier B.V.Citation153