Figures & data
Table 1 Peptide sequence used for assembling gold nanoparticle- PEG conjugates
Table 2 Characterization of gold nanoparticles before and after peptide modification
Figure 1 Localization of GNP-PEG/SV40 at the nuclear membrane and morphological changes to the nuclear pore complex. (A) A fluorescent probe (10 × thymin-FITC) was intermixed with PEG and signal peptides (SV40 and ADV) on the surface of gold nanoparticles and visualized by confocal microscopy (green color). GNP-PEG/SV40 accumulated around the nuclear membrane (Z axis section), whereas unmodified particles were distributed in the cytoplasm after 24 hours of treatment. (B) Due to the high contrast properties of the gold nanoparticles, accumulated particles (dark spots) were observed for the differential interference contrast channel with the same cellular distribution as shown by laser confocal microscopy. (C) Transmission electron microscopic images of the treated cells show GNP-PEG/SV40 blocked nuclear pore complex conduits with increasing time, compared with the untreated control cells, in which the nuclear pore complexes are open and unobstructed. The nuclear pores (arrows) and lamina are clearly shown in the image. Concentration of peptide-modified gold nanoparticles, 7.5 nM.
Abbreviations: ADV, adenovirus; DAPI, 4′,6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate; GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen; N, nucleus; C, cytoplasm.
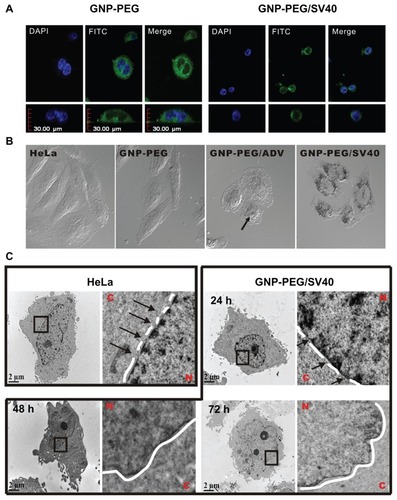
Figure 2 Diminished nuclear pore complex structure after GNP-PEG/SV40 treatment for 72 hours. HeLa cells and nanoparticle-treated HeLa cells were double-labeled with DAPI and α-nuclear pore complex antibody (Mab414), and analyzed by immunofluorescence microscopy. Nuclear rim staining was found in (A) HeLa, (B) GNP-PEG, and (C) GNP-PEG/ADV control cells. The intensity of nuclear rim staining was dramatically reduced and diminished after treatment with (D) GNP-PEG/ SV40. Concentration of peptide-modified gold nanoparticles was 7.5 nM.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; GNP, gold nanoparticle; NPC, nuclear pore complex; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen.
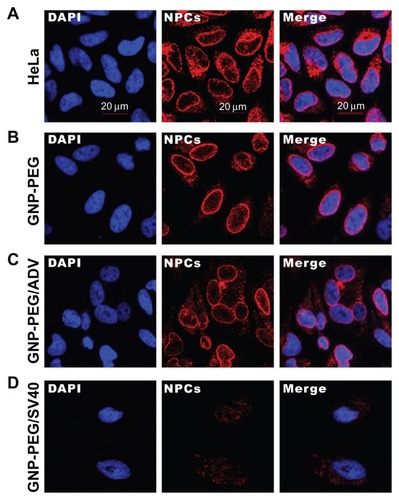
Figure 3 Subcellular distribution of total RNA in cells treated with GNP-PEG, GNP-PEG/ADV, and GNP-PEG/SV40 for (A) 3, (B) 6, and (C) 9 hours. RNA was stained with acridine orange (yellow color) and observed by confocal microscopy. Total RNA was gradually restricted inside the nucleus (DAPI, blue color) with GNP-PEG/SV40 treatment, but not with GNP-PEG or GNP-PEG/ADV (throughout the cytoplasm and nucleus).
Abbreviations: ADV, adenovirus; DAPI, 4′,6-diamidino-2-phenylindole; GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen.
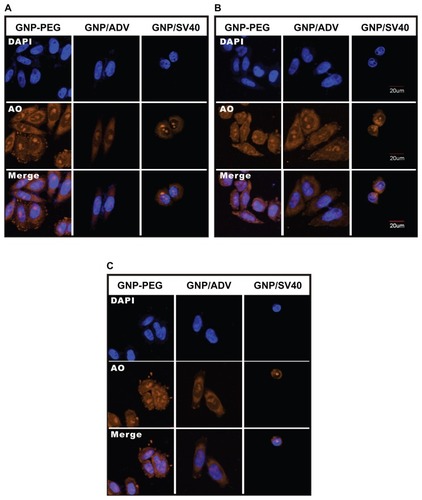
Figure 4 Cellular transportation of shuttle protein STAT1 and transcription regulator NF-κB (red color: Alexa Fluor 594-conjugated rabbit secondary antibody) were both restricted to the cytoplasm due to nucleocytoplasmic transport blockade. (A) IFN-γ and tumor necrosis factor-alpha were used to stimulate the translocation of cytoplasmic STAT1 and NF-κB into the nucleus, respectively, upon GNP-PEG/ADV administration. (B) In contrast, both signaling proteins remained in the cytoplasm and nucleocytoplasmic transport was blocked by GNP-PEG/ SV40, abolishing the related signal cascade. Concentration of peptide-modified gold nanoparticles was 7.5 nM.
Abbreviations: ADV, adenovirus; DAPI, 4′,6-diamidino-2-phenylindole; GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen; IFN-γ, interferon gamma; STAT1, signal transducer and activator of transcription 1; NF-κB, nuclear factor kappaB; TNF-α, tumor necrosis factor alpha.
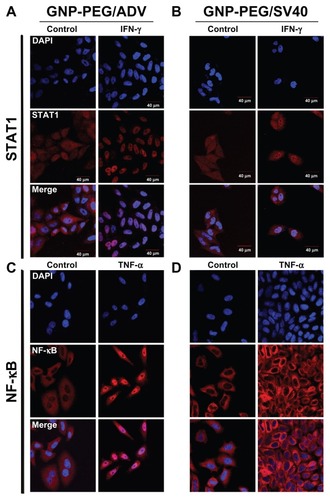
Figure 5 Cell death was caused by administration of GNP-PEG/SV40 at (A) different gold concentrations (B) for different time periods. Transmission electron microscopic images of GNP-PEG/SV40-treated cells exhibiting small vesicles at (C) 24 and (D) 72 hours, which eventually caused the dissociation of cellular apparatus by digestion vesicles, such as autophagosomes (e-al) (arrows) and autolysosomes (d-al) (arrow head).
Abbreviations: ADV, adenovirus; GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen.
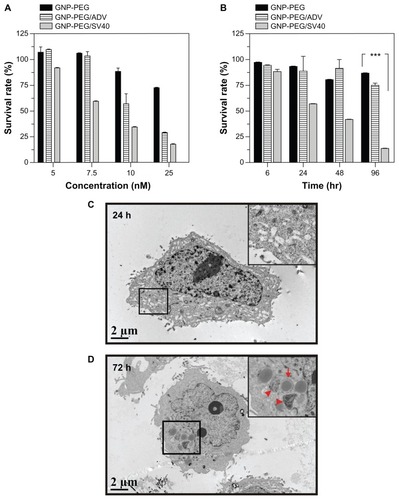
Figure 6 Autophagic cell death was induced by administration of GNP-PEG/SV40. (A) Immunoblotting for LC3-I and its cleaved form LC3-II using total cell lysates from HeLa cells treated with different concentrations (5.0 to 25 nM) of peptide-modified GNP ranged and (B) at a concentration of 7.5 nM for 24 hours to 96 hours. Quantitative results for LC3-II production were normalized to β-actin protein and compared with the HeLa cells for three independent experiments. (C) EGFP-fused LC3 accumulation observed in GNP-PEG/SV40-treated HeLa cells. HeLa cells were transiently transfected with plasmid encoding EGFP-LC3, incubated for 24 hours, and then treated with peptide-modified GNP for 48 hours. Cytoplasmic LC3 vacuoles (green spots) only occurred in GNP-PEG/SV40-treated cells, and 3-methyl adenine, an inhibitor of autophagy, prevented LC3-II formation.
Notes: Data are presented as the mean ± standard error of the mean. **P < 0.01; ***P < 0.001.
Abbreviations: ADV, adenovirus; DAPI, 4′,6-diamidino-2-phenylindole; EGFP, enhanced green fluorescent protein; GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen; LC3, microtubule-associated protein 1 light chain 3.
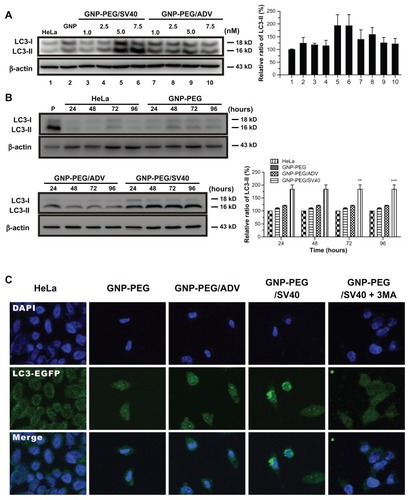
Figure 7 Autophagic cell death was induced in HeLa cells by the nuclear pore complex inhibitor wheat germ agglutinin. (A) Cytotoxicity of wheat germ agglutinin at different concentrations (0.05, 0.5, 5.0, 25 and 50 μg/mL) was measured in HeLa cells using the MTT assay. (B) Western blot for the autophagic marker LC3-II after wheat germ agglutinin treatment at different doses (0.05, 0.5, 5.0, 25, and 50 μg/mL) and for different time periods (24 to 96 hours).
Note: The results shown are the mean ± standard error of the mean of triplicate determinations from one of three identical experiments.
Abbreviations: WGA, wheat germ agglutinin; PBS, phosphate-buffered solution; LC3, microtubule-associated protein 1 light chain 3.
Figure 8 Knockdown of Beclin-1 (BECN1) by small hairpin RNA attenuates GNP-PEG/SV40 induced autophagic cell death. (A) Expression of BECN1 protein was successfully knocked down in HeLa cells. (B) Immunoblotting for LC3-II formation using total cell lysates from HeLa and BECN1-negative cells treated with each peptide-modified GNP at a concentration of 5.0 and 7.5 nM. (C) After GNP-PEG/SV40 (7.5 nM) treatment for different time periods (24 to 96 hours), cell death was significantly attenuated in BECN1-negative HeLa cells compared with control cells.
Notes: Data are shown as the mean ± standard error of the mean. ***P < 0.001.
Abbreviations: ADV, adenovirus; GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen.
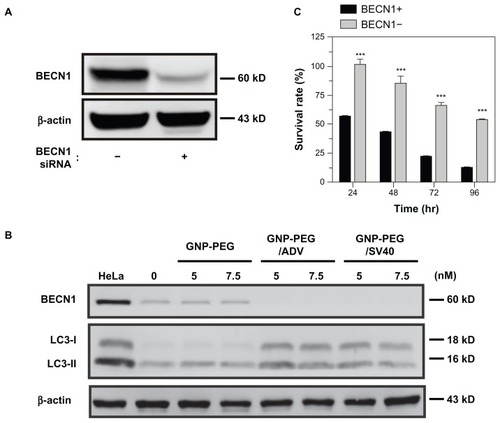
Figure S1 Cellular uptake of GNP-PEG/ADV for different time points. (A) After 6 hours of incubation, only a small amount of GNP-PEG/ADV was capable of targeting the nuclear compartment in aggregated (white circle) or dispersed form (arrow). (B) It was then exported from the nucleus after 12 hours of treatment. (C) After 48 hours, a large proportion of GNP-PEG/ADV was grouped inside the small vesicles (protein body). No nuclear membrane accumulation or nucleocytoplasmic transport blockade was found in the control group.
Abbreviations: ADV, adenovirus; GNP, gold nanoparticle; PEG, poly(ethylene glycol); N, nucleus; C, cytoplasm.
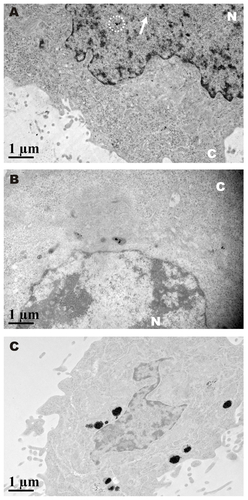
Figure S2 Cellular localization of different peptide-modified gold nanoparticles was analyzed by laser confocal microscopy in SiHa cells. GNP-PEG/SV40 accumulated focally in the perinuclear area of the cytoplasm, but only translocalized into the nucleus in the GNP-PEG/ADV-treated group.
Abbreviations: ADV, adenovirus; GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen.
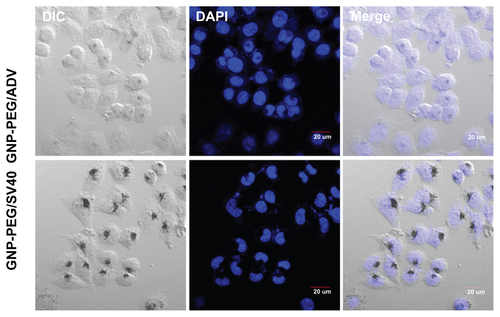
Figure S3 Cellular localization of tRNALys in HeLa cells treated with GNP-PEG/SV40 and wheat germ agglutinin (WGA). Quantitative reverse transcriptase polymerase chain reaction were performed with nuclear RNA isolated from GNP-PEG/SV40 (7.5 nM) and WGA treated HeLa cells. Increased nuclear accumulation of tRNALys confirmed nucleocytoplasmic transport blockade in HeLa cells treated with GNP-PEG/ SV40 and WGA.
Abbreviations: GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen.
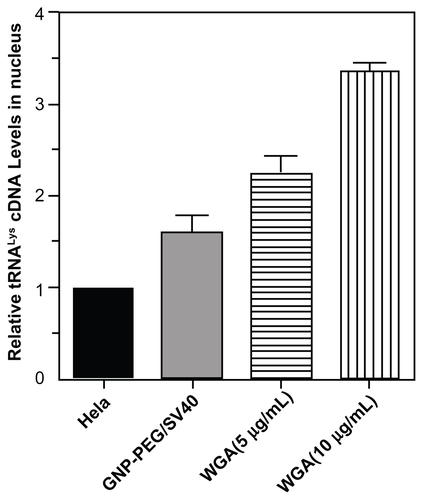
Figure S4 Quantification of peptide-modified gold nanoparticle internalization via atomic absorption spectrometry. Analysis of GNP-PEG, GNP-PEG/ADV, and GNP-PEG/SV40 uptake by HeLa cells exposed to 7.5 nM nanoparticles for 24 hours. No significant difference in uptake was found between GNP-PEG/ADV-treated and GNP-PEG/SV40-treated cells (GNP-PEG/ADV, 4.041 ± 0.136 μg Au/105 cells; GNP-PEG/ SV40, 4.358 ± 0.253 μg Au/105 cells).
Abbreviations: ADV, adenovirus; GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen.
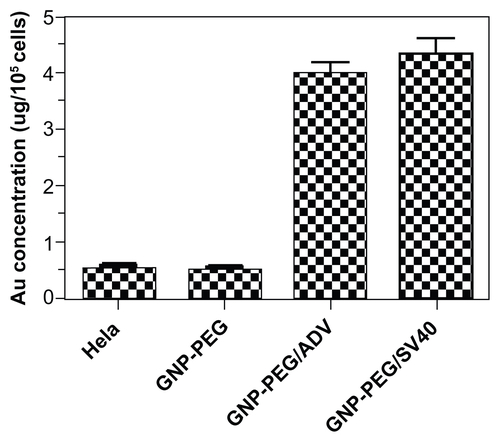
Figure S5 GNP-PEG/SV40-induced cell death did not involve the caspase-dependent mechanism of apoptosis. Cells treated with peptide-modified gold nanoparticles (72 μg/ mL) were harvested at different times (24, 48, 72, and 96 hours after treatment) and were immunoblotted for caspase-9 and activated caspase-3. β-Actin was used as the loading control. P refers to positive controls (cisplatin-treated HeLa cells). No detectable activated caspase protein was found within the treated cells, indicating the death mechanism was not via the apoptosis signaling pathway.
Abbreviations: ADV, adenovirus; GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen.
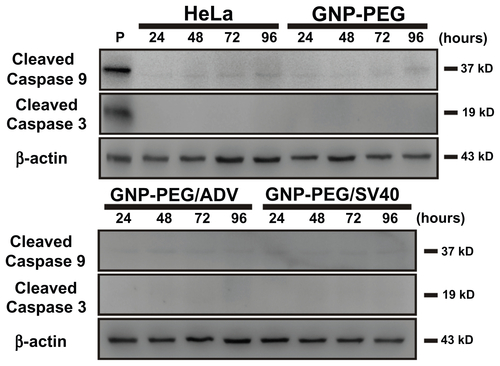
Figure S6 GNP-PEG/SV40-induced cell death did not involve the mechanism of necrosis and early apoptosis. Flow cytometric profiling of annexin-V/propidium iodide double staining shows necrotic cells in the top right quadrant, apoptotic cells in the lower right quadrant, and viable cells in the bottom left quadrant. During treatment with GNP-PEG/ SV40, the annexin-V/propidium iodide staining was similar to that of the HeLa cell control located in the bottom left quadrant at (A) various concentrations (9.6, 24, 48 and 72 μg/mL) and (B) time periods (24 hours to 96 hours) of treatment. P refers to positive controls (cisplatin-treated HeLa cells at 50 μM for 24 hours).
Abbreviations: ADV, adenovirus; GNP, gold nanoparticle; PEG, poly(ethylene glycol); PI, propidium iodide; SV40, simian virus 40 large T antigen.

Figure S7 Autophagic cell death was not induced by GNP-PEG/SV40 in SiHa cells. Immunoblotting for LC3-I and its cleaved form LC3-II using total cell lysates (5 μg) from SiHa cells at (A) different concentrations of peptide-modified GNP ranging from 9.6 to 72 μg/mL and (B) at the concentration of 72 μg/mL for 24 hours to 96 hours.
Abbreviations: ADV, adenovirus; GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen; LC3, microtubule-associated protein 1 light chain 3.
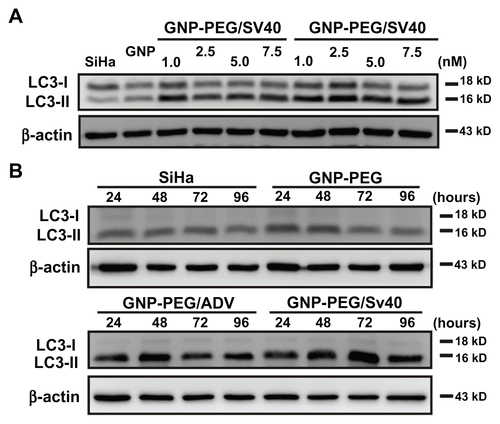
Figure S8 Autophagic cell death was induced by the nuclear pore complex inhibitor, wheat germ agglutinin (WGA), in SiHa cells. The autophagic marker, LC3- II, was probed by Western blotting of WGA given at different doses (0.05, 0.5, 5.0, 25, and 50 μg/mL) and time periods (24 to 96 hours). After treatment, autophagy increased significantly at 5.0, 25, and 50 μg/mL concentrations and was expressed from 24 hours to 96 hours.
Abbreviations: LC3, microtubule-associated protein 1 light chain 3; PBS, phosphate-buffered saline.
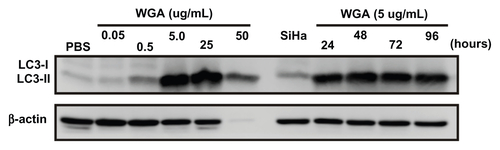
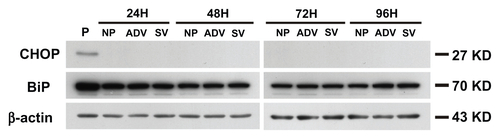
Figure S9 The cell death mechanism of GNP-PEG/SV40-mediated nucleocytoplasmic transport blockade was not via the endoplasmic reticulum stress pathway. The effects of each group throughout different time periods on the relative intracellular levels of CHOP and BiP were assessed by Western blot analysis. Whole cell lysates were collected for the assay. β-Actin was visualized to ensure equal loading in each lane. The expressed level of CHOP was undetectable in each group and without any change of BiP under treatment.
Abbreviations: ADV, adenovirus; BiP, immunoglobulin heavy chain binding protein in pre-B cell; CHOP, CCAAT-enhancer binding protein homologous protein; GNP, gold nanoparticle; PEG, poly(ethylene glycol); SV40, simian virus 40 large T antigen; P, positive control.