Figures & data
Table 1 Chemical Structure of the Biomaterials Discussed in This Article
Figure 1 Scaffolds seeded with cells can be utilized in combination with microcarriers to accelerate angiogenesis. (A) Microcarriers can be modified in terms of porosity/interconnectivity and can contribute to angiogenesis with hydrogels/scaffolds. Using these templates, angiogenesis will be more discernible leading to a more comprehensive understanding of angiogenesis. We can seed different kinds of cells (such as mesenchymal stem cells) and nanoscale materials can greatly support their division and differentiation. (B) Cooperation between polymeric scaffolds with microcarriers provides a strong template for angiogenesis. We can couple the seeded microcarriers with hydrogels and scaffolds (polymeric or natural) which provide a good microenvironment for cell growth. (C) Hydrogels can be used for cell seeding or cell encapsulation and microcarriers can be combined with them for sustained drug or biomolecule delivery. (D) Using the above-mentioned information, cell seeding in acellular scaffolds can lead to tissue angiogenesis. (E) A complete engineered tissue with an expansive vasculature network is the last large step forward. Created with Procreate Software.
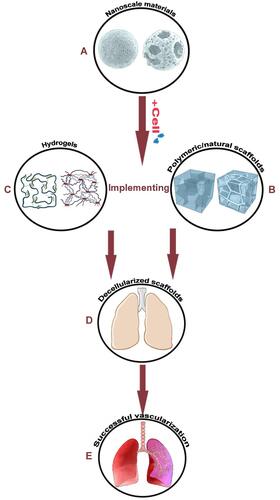
Figure 2 In vivo applications of a Gel−POSS hybrid hydrogel primed using octafunctional POSS cages. Cell adhesion, controlled release of growth factors, repairing tissue defects, as well as the acceleration of angiogenesis are some of the uses of this platform. Reprinted with permission from Chen M, Zhang Y, Zhang W, Li J. Polyhedral oligomeric silsesquioxane-incorporated gelatin hydrogel promotes angiogenesis during vascularized bone regeneration. ACS Appl Mater Interfaces. 2020;12(20):22410–22425. Copyright 2022 American Chemical Society.Citation74
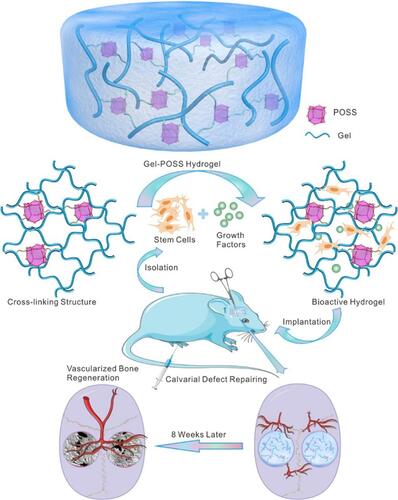
Table 2 A Review of Templates Fabricated for Angiogenesis
Figure 3 Schematic of prepared 3D fibrous scaffolds in conjunction with applied biochemical and biomechanical stimuli leading to a positive effect on angiogenesis and osteogenesis for bone repair applications. Reprinted with permission from Kim JJ, El-Fiqi A, Kim HW. Synergetic cues of bioactive nanoparticles and nanofibrous structure in bone scaffolds to stimulate osteogenesis and angiogenesis. ACS Appl Mater Interfaces. 2017;9(3):2059–2073. Copyright 2022 American Chemical Society.Citation90
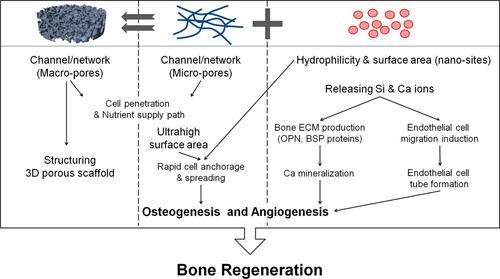
Figure 4 BMP-2 and VEGF release sequences from gelatin and 2-N,6-O-sulfated chitosan scaffolds. The efficient binding of VEGF to SCS is the reason behind the observed synergistic angiogenesis. Reprinted with permission from Zhang S, Chen J, Yu Y, Dai K, Wang J, Liu C. Accelerated bone regenerative efficiency by regulating sequential release of BMP-2 and VEGF and synergism with sulfated chitosan. ACS Biomater Sci Eng. 2019;5(4):1944–1955. Copyright 2022 American Chemical Society.Citation105
Figure 5 (A) First two rows show the in ovo results of blood vessel formation for cholecyst-derived scaffolds and cholecyst-derived scaffolds-gelatin and the third row, shows ex ovo results. (B) Stereomicroscopic images of vessels for both cholecyst-derived scaffolds and cholecyst-derived scaffolds-gelatin (Scale bars are in the range of 1 to 2 µm). (C) Illustration of “vascular index” which reports the angiogenesis acceleration after the addition of gelation to cholecyst-derived scaffolds. (CAM= Chick Chorioallantoic Membrane, *p value < 0.05). Reprinted with permission from Mony MP, Shenoy SJ, Raj R, et al. Gelatin-Modified Cholecyst-Derived Scaffold Promotes Angiogenesis and Faster Healing of Diabetic Wounds. ACS Appl Bio Mater. 2021;4(4):3320–3331. Copyright 2022 American Chemical Society.Citation107
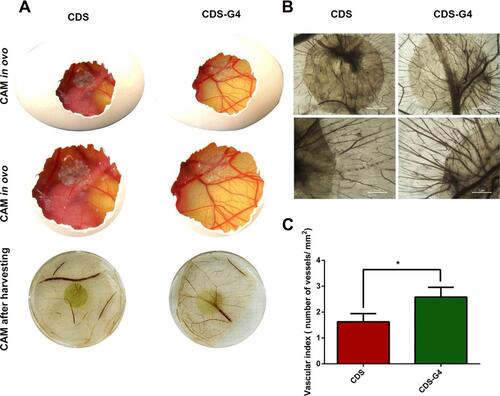
Figure 6 Depiction of dual cross-linked high-molecular-weight hyaluronic acid seeded hydrogels with M2 phenotype macrophages improving immunocompromization and defecting angiogenesis. Reprinted with permission from Liu S, Yu J, Zhang Q, et al. Dual cross-linked HHA hydrogel supplies and regulates MΦ2 for synergistic improvement of immunocompromise and impaired angiogenesis to enhance diabetic chronic wound healing. Biomacromolecules. 2020;21(9):3795–3806. Copyright 2022 American Chemical Society.Citation115
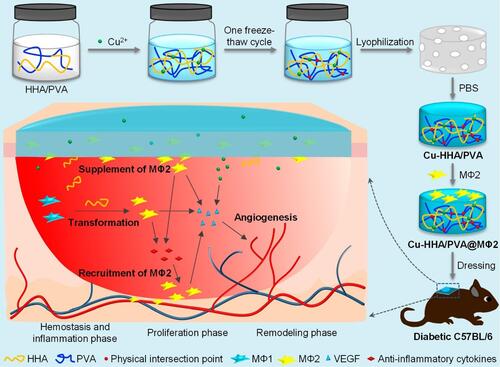
Table 3 A Review of Templates Fabricated for Angiogenesis
Figure 7 An example of in vitro 3D cell encapsulation within a hydrogel leading to cell proliferation, and migration. (A) acylated-modified sulfobetaine-derived starch (SB-ST-A), (B) dithiol-functionalized poly (ethylene glycol) (PEG-SH), (C) cells, and (D) cell-laden hydrogel. Reprinted with permission from Dong D, Li J Cui M, et al. In situ “clickable” zwitterionic starch-based hydrogel for 3D cell encapsulation. ACS Appl Mater Interfaces. 2016;8(7):4442–4455. Copyright 2022 American Chemical Society.Citation128
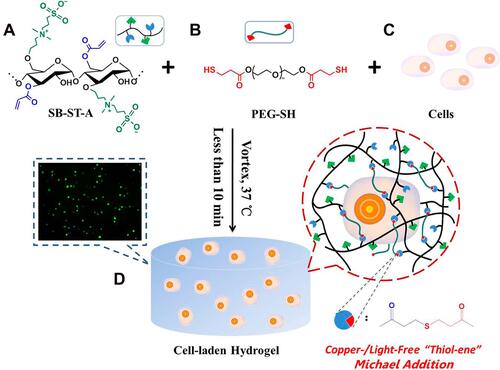
Figure 8 The therapeutic influence of MSCs-biomaterials due to the release of trophic factors, specifically immunomodulatory or angiogenic cytokines. Reprinted with permission from Li T, Ma H, Ma H, et al. Mussel-inspired nanostructures potentiate the immunomodulatory properties and angiogenesis of mesenchymal stem cells. ACS Appl Mater Interfaces. 2019;11(19):17134–17146. Copyright 2022 American Chemical Society.Citation145
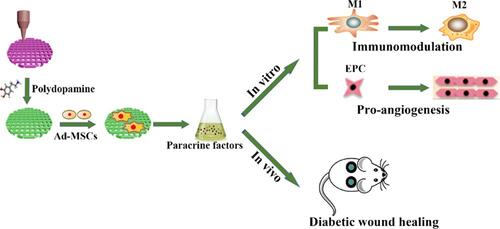
Table 4 Different Cell Sources That Can Be Seeded for Enhancing Angiogenesis
Table 5 Effect of Mechanical Factors on Angiogenesis
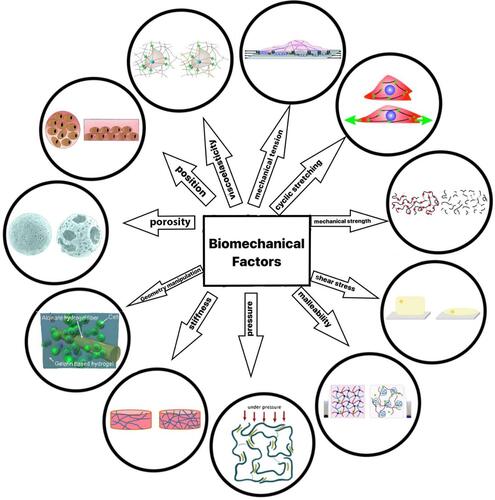
Figure 9 Different biomechanical stimuli for angiogenesis expansion. Created with Procreate Software