Figures & data
Table 1 Average number of magnetic nanoparticles incorporated within a single cell as a function of incubation time, as calculated from the saturation magnetization of magnetically loaded cells
Figure 1 Number of magnetic nanoparticles uploaded per cell as a function of incubation time.
Notes: The observed decrease of incorporated magnetic nanoparticles followed an exponential decay, and reached a near-steady state for incubation times longer than 1 hour.
Abbreviation: NP, nanoparticles.
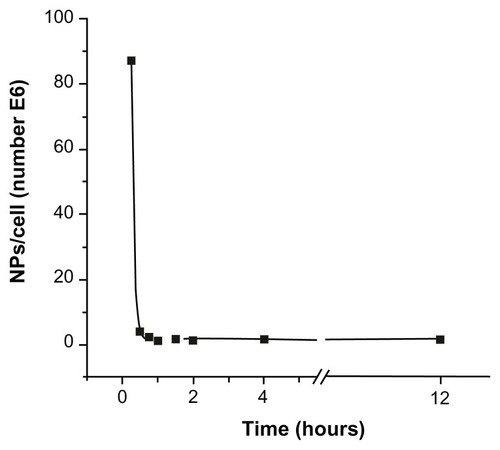
Figure 2 Schematic view of the experimental 2 × 2 design for evaluating the effect of magnetic nanoparticles and time-varying magnetic fields on Crithidia fasciculata. (A) Cells without magnetic nanoparticles not submitted to magnetic fields. (B) Cells with magnetic nanoparticles without magnetic field application. (C) Application of magnetic fields on unloaded cells. (D) Application of magnetic fields on magnetic nanoparticle-loaded cells.
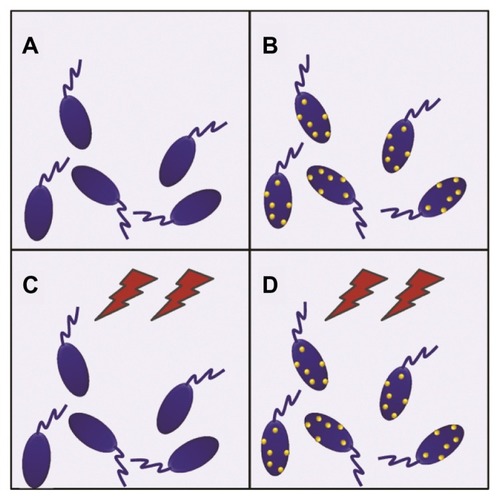
Figure 3 MTT assay results for the four conditions displayed in .
Notes: All samples showed 100% of cell viability except in the case of time-varying magnetic fields applied on magnetically loaded cells, which caused 95% ± 5% cell death.
Abbreviations: MNPs−/TVMF−, cells not-bearing MNPs that were not submitted to TVMF; MNPs+/TVMF−, cells bearing MNPs that were not submitted to magnetic field application; MNPs−/TVMF+, cells not bearing MNPs that were submitted to magnetic field application; MNPs+/TVMF+, cells bearing MNPs that were submitted to magnetic field application; MNPs, magnetic nanoparticles; TVMF, time-varying magnetic field; MTT, (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
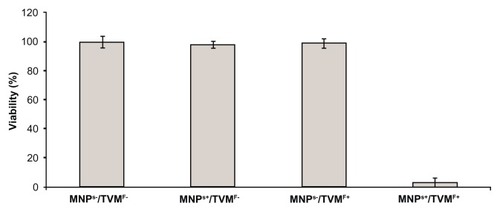
Figure 4 Scanning electron microscopy images of MNPs+ (magnetic nanoparticles)/TVMF+ (time-varying magnetic field) sample before (A) and after (B) application of magnetic fields.
Note: In the latter case, the changes in cell morphology can be clearly observed, reflecting the severe cell damage after TVMF.
Figure 5 Specific power absorption of magnetic colloid (solid squares) at 1% weight concentration, and the nanoparticle-loaded protozoa (solid line) during application of AC magnetic field (H = 160 Oe, f = 250 kHz).
Abbreviations: MAN, magnetic colloid; C. fasciculata, Crithidia fasciculata; T, temperature; t, time.
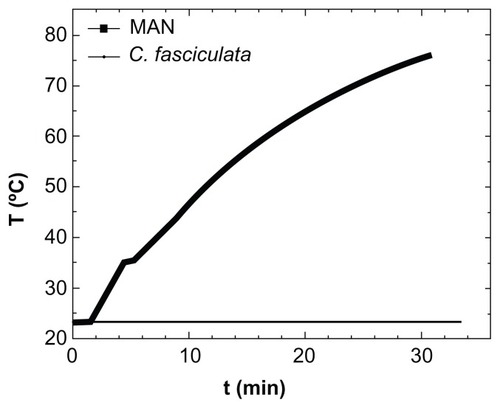
Figure 6 Flow cytometry results of the 2 × 2 experiments shown in (see text for details). Experiments (A–C) showed 89%–91% cell viability, whereas for experiment (D), application of magnetic fields for 30 minutes on magnetically charged cells resulted in only 9% cell survival.
Abbreviations: P1-A, propidium iodide; FITC, fluorescein isothiocyanate.
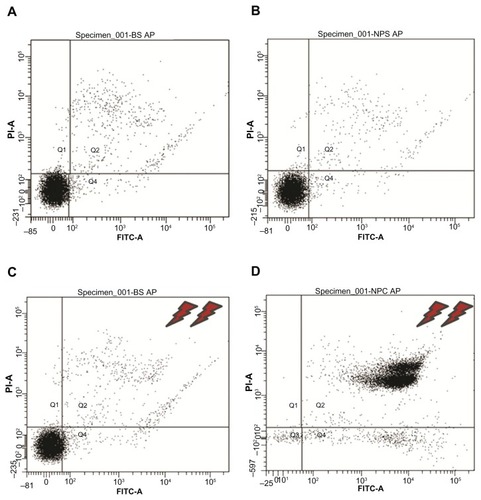
Figure S1 Magnetic response at T = 10 K from (A) unloaded cells, (B) response from Crithidia fasciculata cocultured with MNPs (sample incubated for 15 minutes), (C) difference between loaded and unloaded cells (B − A), and (D) pure magnetic colloid. Note that for the pure colloid (D), the curve was divided by 1.35 × 104 to fit the same scale as the magnetic signal from loaded cells (C).
Notes: To calculate the amount of magnetic material mmag incorporated by the cells, the MS values from the pure colloids and from the magnetic nanoparticle-loaded cells were calculated as mmag [g/cell]=M/MS× Number of cells. The number of magnetic nanoparticles per single cell was estimated from the known average particle diameter.
![Figure S1 Magnetic response at T = 10 K from (A) unloaded cells, (B) response from Crithidia fasciculata cocultured with MNPs (sample incubated for 15 minutes), (C) difference between loaded and unloaded cells (B − A), and (D) pure magnetic colloid. Note that for the pure colloid (D), the curve was divided by 1.35 × 104 to fit the same scale as the magnetic signal from loaded cells (C).Notes: To calculate the amount of magnetic material mmag incorporated by the cells, the MS values from the pure colloids and from the magnetic nanoparticle-loaded cells were calculated as mmag [g/cell]=M/MS× Number of cells. The number of magnetic nanoparticles per single cell was estimated from the known average particle diameter.](/cms/asset/d61c54a6-7b4f-4c3a-a664-b1089f5bc959/dijn_a_35510_sf0001_c.jpg)