Figures & data
Figure 1 The source of MSCs, the process of secreting MSC-Exos, and the mechanism of interaction between MSC-Exos and recipient cells. MSCs can be isolated from fat, placenta, bone marrow, and muscle tissue. As the plasma membrane buds inward, the secreted proteins form early endosomes through endocytosis, followed by late endosomes, and finally multivesicular bodies (MVBs). Some MVBs release vesicles into the extracellular space as exosomes. MSC-Exos can deliver cargo to recipient cells in three ways: endocytosis, direct membrane fusion, and receptor ligand binding.
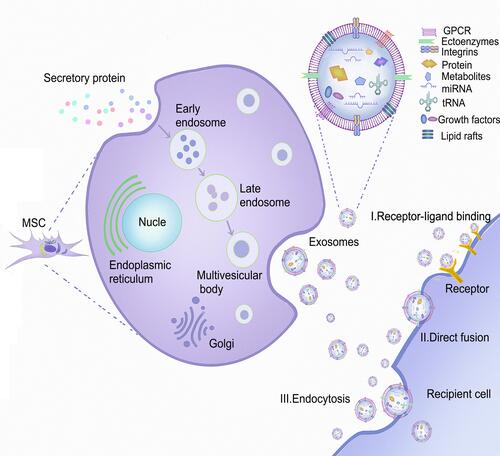
Figure 2 The homing process of MSCs in the inflammatory microenvironment: (1) tethering and rolling; (2) activation; (3) arrest; (4) transmigration or diapedesis; and (5) migration. The figure also shows the interaction of MSCs with endothelial cells during the homing process, and the chemokine released at the injury site guides the migration of MSCs in the extracellular matrix.
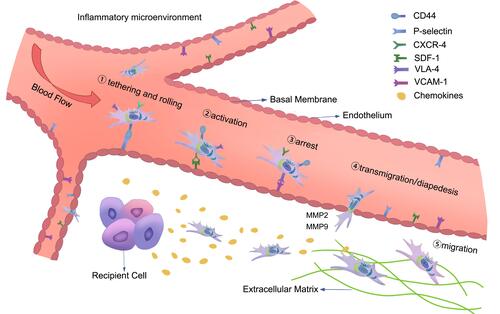
Figure 3 The mechanism of MSCs in an inflammatory environment. Arrows indicate activation or induction, and T-bars indicate inhibition. MSCs that differentiate into different lineages of cells and regenerate damaged tissues can inhibit T cells, B lymphocytes, neutrophils, natural killer cells (NK cells) and mature DC cells by secreting cytokines or growth factors, so as to regulate inflammation and promote the transformation of monocytes and M1 macrophages into M2 macrophages. At the same time, IL-10 produced by M2 macrophages can promote the formation of Treg and reduce the migration of neutrophil tissue.
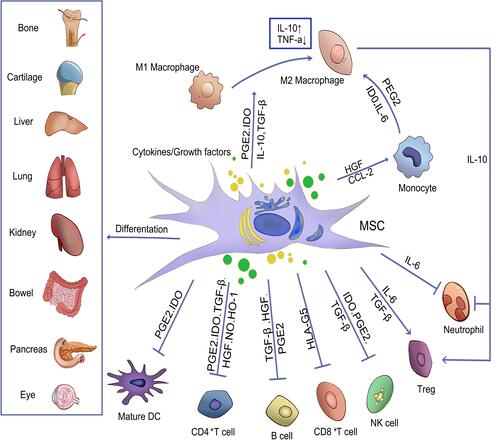
Table 1 Summary of Some Clinical Trials Based MSCs in Inflammatory Diseases
