Figures & data
Table 1 Classification of QDs According to Their Chemical Composition
Figure 1 A scheme of some commonly-used methods for the preparation of QDs. (A) Colloidal synthesis. Precursors are injected into organic or aqueous system under high temperature to facilitate their conversion into the molecular state, with a subsequent assembly into QDs. The major reaction steps include nucleation, crystal growth, and termination, which can be manipulated to control the physico-chemical properties of the produced QDs. (B) Biotemplate-based synthesis. Biological entities such as bacteriophages, genetically-engineered viruses, DNA, or peptides are used as templates to assemble the precursors into QDs. (C) Electrochemical assembly. Electrochemical driving force is used to assemble the precursor ions into QDs at the electrolyte-metal interface. (D) Biogenic synthesis. Heavy metal ions are detoxified via binding to cysteine-terminated peptides, followed by their introduction into microorganisms such as E. coli, where they react with the endogenous co-precursors (eg, sulfide ions) and assemble into QDs, which are subsequently exported out of the microorganism. Created by BioRender.com.
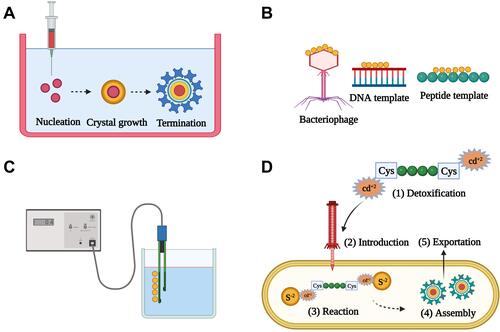
Table 2 Impact of Core Composition on the Particle Size and Emission Spectra of QDs.Citation44
Figure 2 Impact of the particle size of CdSe QDs on their emission spectra upon irradiation with UV light. The emission wavelength is directly proportional to the particle size. Adapted from Bera D, Qian L, Tseng T-K, Holloway PH. Quantum Dots and Their Multimodal Applications: A Review. 2010;3(4):2260–2345.Copyright © 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).Citation43
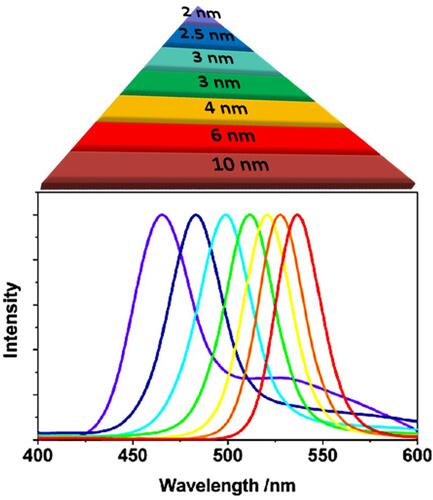
Figure 3 Some biomedical applications of QDs. (A) Intracellular imaging. QDs decorated with specific targeting moieties are used as fluorescent labels for intracellular visualization by fluorescence microscopy or confocal laser scanning microscopy (CLSM). (B) In vivo imaging. Following administration, QDs modified with tissue-specific targeting moieties can be used for visualization of certain organs in question using in vivo imaging systems (IVIS). (C) Fluorescence-activated cell sorting (FACS). QDs decorated with cell-specific ligands can be used as fluorescent probes for cell sorting during flow cytometry. Thanks to numerous advantages, QDs have a better capacity in polychromatic cell sorting compared to conventional organic dyes. (D) Photodynamic therapy (PDT). Following irradiation, QDs can act as photosensitizers or energy donors to other photosensitizers to generate reactive oxygen species (ROS) in situ leading to apoptotic cell death during cancer treatment. (E) Traceable drug delivery vehicles. QDs can be used as drug carriers to various tissues with high extravasation and tissue penetration capabilities thanks to their ultra-fine particle sizes. Owing to their quantum properties, QDs accumulation in the target tissues can be easily tracked. Created by BioRender.com.
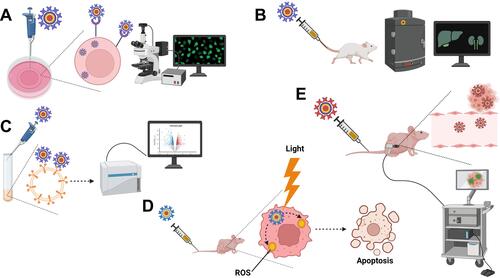
Table 3 Examples of Functional Surface Modifications of QDs and Their Applications
Figure 4 Some common routes by which functionalized QDs internalize into the target cells. (A) Clathrin-mediated endocytosis. Following concentration of QDs at the receptor region, QDs are engulfed into clathrin-coated cell membrane invaginations, forming clathrin-coated vesicles. Dynamin mediates the dissociation of the aforementioned vesicles from the cell membrane into the cytosol leading to the formation of endosome. Subsequently, QDs should escape from the endosome and be released into the cytosol to avoid lysosomal degradation. (B) Caveolae-mediated endocytosis. The bindings of functionalized QDs to certain receptors mediates their internalization into the target cells via cholesterol-rich flask-shaped membrane invaginations, called caveolae, which subsequently dissociate from the cell membrane, forming caveosomes. Caveosomes are thought to be less destructive than endosomes. (C) Macropinocytosis. The binding of functionalized QDs to certain receptors activates the formation of cell membrane ruffles that engulf QDs into the cytosol forming macropinosomes, which subsequently leak their cargo into the cytosol. Created by BioRender.com.
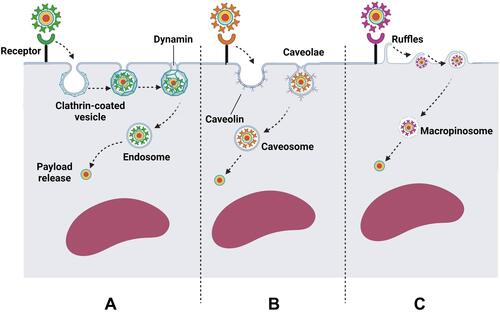
Table 4 Clinical Trials Recruiting QDs as of December 2021Citation87
Figure 5 Common challenges hampering the clinical translation of QDs. Stability issues include the high liability for degradation and aggregation during storage. Industrial issues arise from the complex scale-up procedures and the environmental hazards rendered by the heavy metal components. In vivo issues include the substantial loss of the injected dose via renal clearance owing to the ultra-fine particle size, poor selectivity due to non-specific interactions with tissues and cellular membranes, and intracellular toxicity caused by the generation of ROS or DNA damage. Created by BioRender.com.
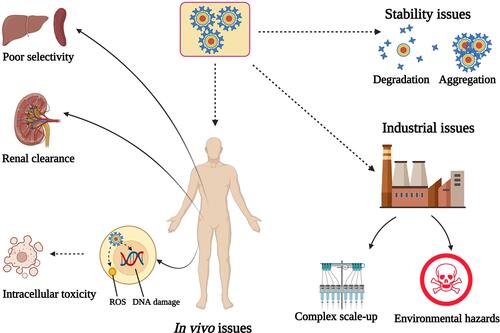
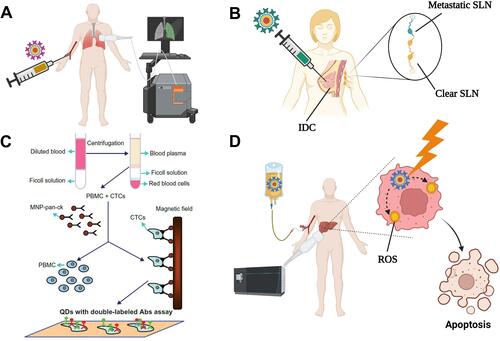
Figure 6 Some potential applications of QDs in clinics. (A) Diagnostic imaging. QDs modified with specific targeting ligands can be injected intravenously to accumulate into the target organ, allowing its visualization. (B) Sentinel lymph node (SLN) detection. QDs can substitute the currently-used blue dye or radionuclide methods for the intraoperative detection of SLNs. Following peritumoral injection, QDs diffuse to the affected SLN(s) allowing accurate and safe detection of SLNs metastases. (C) Detection of micrometastases. Blood samples from cancer patients are fractioned by gradient centrifugation to collect PBMC and CTCs, followed by their mixing with magnetic nanoparticles that are labeled with anti-pan-ck antibody to enrich lung cancer epithelial cells. The micrometastatic cells conjugated to the magnetic nanoparticles are separated using a magnetic field, incubated with double antibody-labelled QDs, and detected under a fluorescence microscope. The figure (6C) is reproduced from Wang Y, Zhang Y, Du Z, Wu M, Zhang G. Detection of micrometastases in lung cancer with magnetic nanoparticles and quantum dots. Int J Nanomedicine. 2012;7:2315–2324. With a permission from Dove Medical Press, copyright 2012.Citation81 (D) Cancer photodynamic therapy. Following administration of QDs, they tend to accumulate into the tumor site via passive or active targeting. Local irradiation of the tumor site induces the generation of ROS that kill the tumor cells. Created by BioRender.com.