Figures & data
Table 1 Physicochemical properties of the baohuoside I-phospholipid complex (n = 3)
Figure 2 Transmission electron microscopic images of baohuoside I-phospholipid complexes of different sizes. (A) 262 ± 24 nm, (B) 148 ± 12 nm, and (C) 81 ± 10 nm.
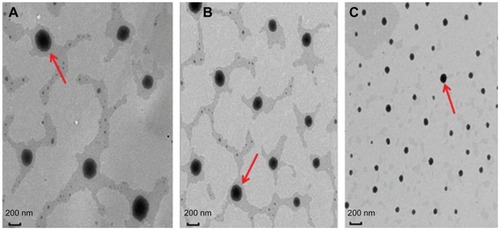
Figure 3 Chromatograms of (A) baohuoside I and (B) a nanoscale baohuoside I-phospholipid complex (81 ± 10 nm).
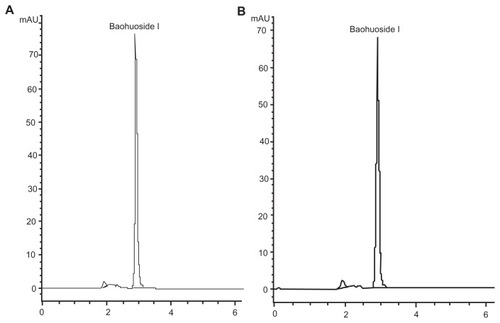
Figure 4 Ultraviolet spectra of (A) baohuoside I and (B) a nanoscale baohuoside I-phospholipid complex (81 ± 10 nm).
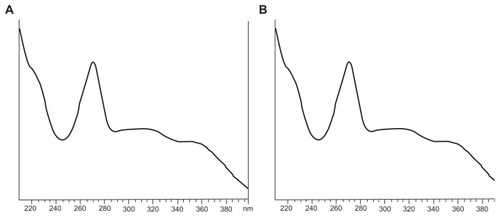
Figure 5 Differential scanning calorimetry thermograms of (A) baohuoside I, (B) the phospholipids, (C) the physical mixture, and (D) the nanoscale baohuoside I-phospholipid complex.
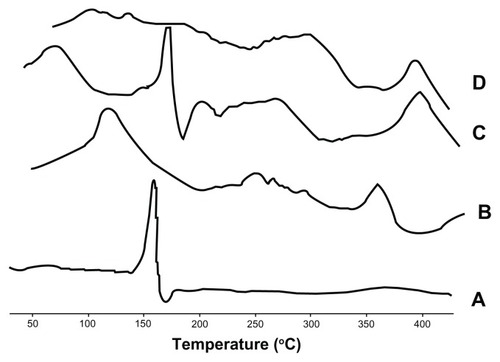
Figure 6 Permeability of (A) baohuoside I, (B) the baohuoside I-phospholipid complex of 262 ± 24 nm, (C) the baohuoside I-phospholipid complex of 148 ± 12 nm, and (D) the baohuoside I-phospholipid complex of 81 ± 10 nm.
Notes: The rates of transport were used to calculate the absorptive permeability [Papp (A − B)] and secretory permeability [Papp (B − A)] using EquationEquation (1)(1) , and the calculated permeabilities are plotted here as bars. The data are expressed as the mean ± standard deviation (n = 3). *P < 0.05 baohuoside I versus the baohuoside I-phospholipid complex, with more asterisks indicating higher levels of significance (one-way analysis of variance followed by Tamhane’s post hoc test).
![Figure 6 Permeability of (A) baohuoside I, (B) the baohuoside I-phospholipid complex of 262 ± 24 nm, (C) the baohuoside I-phospholipid complex of 148 ± 12 nm, and (D) the baohuoside I-phospholipid complex of 81 ± 10 nm.Notes: The rates of transport were used to calculate the absorptive permeability [Papp (A − B)] and secretory permeability [Papp (B − A)] using EquationEquation (1)Papp=VS×C×dCdt=1S×C×dMdt(1) , and the calculated permeabilities are plotted here as bars. The data are expressed as the mean ± standard deviation (n = 3). *P < 0.05 baohuoside I versus the baohuoside I-phospholipid complex, with more asterisks indicating higher levels of significance (one-way analysis of variance followed by Tamhane’s post hoc test).](/cms/asset/1154bf88-2562-4f1d-8586-899ccf8c815c/dijn_a_35965_f0006_c.jpg)
Table 2 Permeabilities (Papp) and efflux ratios of baohuoside I and the nanoscale baohuoside I-phospholipid complex
Table 3 Pharmacokinetic parameters of baohuoside I, baohuoside I-phospholipid complex of 227.3 ± 65.2 μm (BPC a), baohuoside I-phospholipid complex of 262 ± 24 nm (BPC b), baohuoside I-phospholipid complex of 148 ± 12 nm (BPC c), and baohuoside I-phospholipid complex of 81 ± 10 nm (BPC d) 50 mg/kg, orally in rats (n = 6)
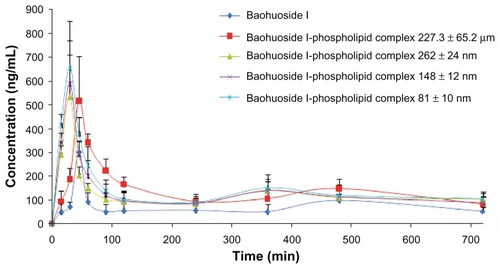
Figure 7 Plasma concentration-time curve in rats after oral administration of baohuoside I and the baohuoside I-phospholipid complexes of 227.3 ± 65.2 μm, 262 ± 24 nm, 148 ± 12 nm, and 81 ± 10 nm.
Notes: The baohuoside I dose was 50 mg/kg. The data are presented as the mean ± standard deviation (n = 6).