Figures & data
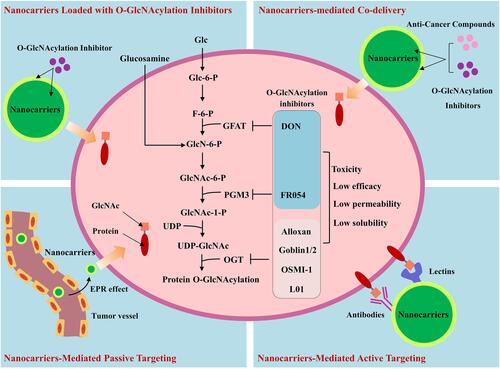
Figure 1 Illustration of the N- and O-link glycosylation, and O-GlcNAcylation. The structures and locations of the canonical N- and O-link glycosylation are compared to those of the non-canonical, cancer-specific O-GlcNAcylation. The N-link glycosylation involves complex combinations of mannose, galactose, and GlcNAc, with a common core composed of 2 GlcNAcs and 3 mannoses. The O-link glycosylation is initiated by the addition of a GalNAc group, and the sugar chains are extended by sequential addition of galactose, GalNAc and GlcNAc. N- and O-link glycosylation are often capped with negatively charged sialic acids. The O-GlcNAcylation is initiated by a GlcNAc, and can be further extended by addition of galactose and sialic acid. Note the intracellular as well as membrane location of O-GlcNAcylation.
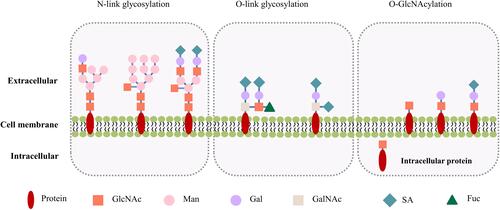
Figure 2 Increased protein O-GlcNAcylation in cancers and therapeutic interference of O-GlcNAcylation. The Warburg effect and an increased glucose/glutamine consumption by cancer cells leads to a high HBP flux and increased UDP-GlcNAc level. This, together with OGT overexpression often causes protein hyper-O-GlcNAcylation in cancer cells. 1) 6-diazo-5-oxo-norleucine (DON) inhibits the glutamine fructose-6-phosphate amidotransferase, and FR054 inhibits the N-acetylglucosamine-phosphate mutase, leading to a reduced intracellular level of UDP-GlcNAc and disruption of O-GlcNAcylation; 2) UDP-GlcNAc analogues or small molecules identified by high throughput screening of compound library, can compete with UDP-GlcNAc upon binding to OGT, and decrease the O-GlcNAcylation level through direct inhibition of OGT activity.
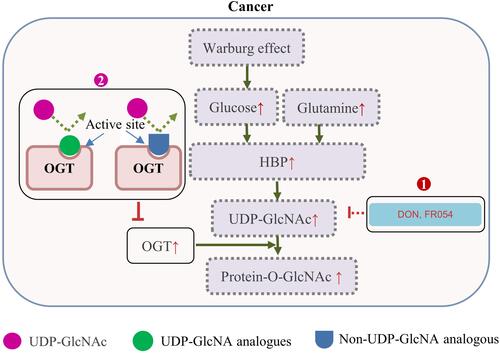
Table 1 Nanoparticles Modified with WGA for Glycosylation Detection or Targeting
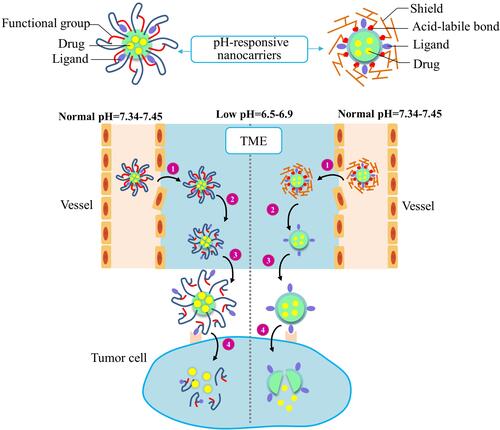
Figure 3 Improved drug delivery efficiency by two types of acidic TME-responsive nanocarriers. The ligands of acidic TME-responsive nanocarriers are covered by either functional groups (Left) or shields (right) before reaching tumor tissues. A nanocarrier covered with functional groups will respond to the low pH by protonation/ionization of the functional groups to reveal the targeting ligands. A nanocarrier covered with shielding molecules will respond to the low pH by degradation/cleavage of the shielding molecules to present the targeting ligands. The two types of nanocarriers undergo these common steps: 1) The EPR effect-promoted penetration and accumulation of nanocarriers in tumor. 2) The low-pH TME-triggered exposure of the targeting ligands. 3) Ligands binding to the cell surface receptors. 4) Internalization of nanocarriers and drug release in cellular compartments.