Figures & data
Table 1 In vivo Antitumor Effect of Free Photosensitizer and MOFs-Based PDT
Figure 3 Characterization and in vivo therapy results of DBC-UiO. (A) The Illustration of DBC-UiO of 1O2 Generation Process; (B) UV-vis absorption spectra of H2DBC, DBC-UiO, H2DBP, and DBP-UiO; (C and D) TEM images of DBC-UiO showing nanoplate morphology (C) before and (D) after incubation in cell culture medium. Reprinted with permission from Lu K, He C, Lin W. A Chlorin-Based Nanoscale Metal-Organic Framework for Photodynamic Therapy of Colon Cancers. J Am Chem Soc. 2015;137(24):7600–7603 Copyright (2015) American Chemical Society.Citation52
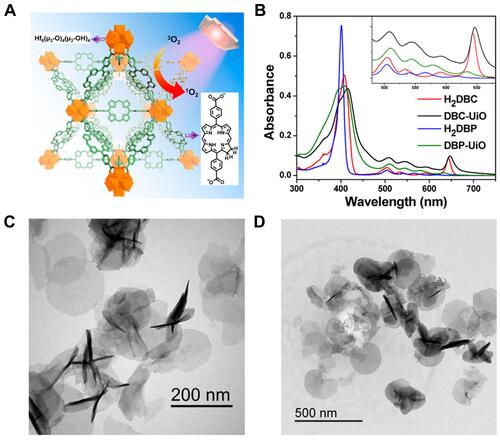
Figure 4 The scheme of TBP-NMOF for inhibition of tumor metastasis. (A) The preparation process of TBP-NMOF; (B) TBP-NMOF-mediated photodynamic cancer immunotherapy. TBP-NMOF-mediated PDT induces an adaptive antitumor immune response that recruits tumor-infiltrating T cells to inhibit tumor metastasis. Reprinted with permission from Zeng JY, Zou MZ, Zhang M, et al. π-Extended Benzoporphyrin-Based Metal–Organic Framework for Inhibition of Tumor Metastasis. ACS Nano. 2018;12(5):4630–4640. Copyright (2018) American Chemical Society.Citation90
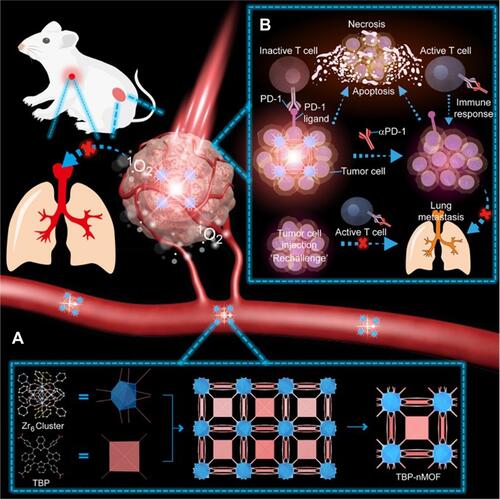
Figure 5 (A) Perspective view of Ti-(Ti·TBP) structure along the (010) direction; (B) coordination environments of Ti-oxo chain SBUs; (C) Schematic showing both type I and type II PDT enabled by Ti-TBP. Reprinted with permission from Lan G, Ni K, Veroneau SS et al. Titanium-Based Nanoscale Metal-Organic Framework for Type I Photodynamic Therapy. J Am Chem Soc. 2019;141(10):4204–4208. Copyright (2019) American Chemical Society.Citation53
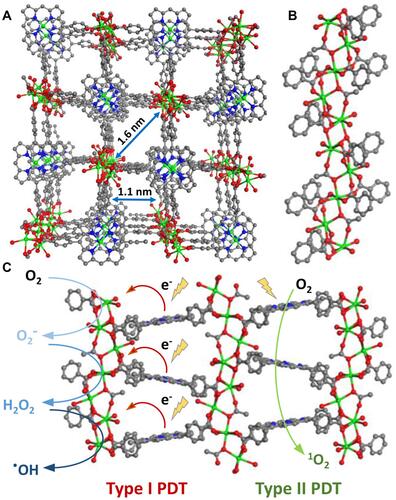
Figure 6 (A) The illustration of bacteriochlorin ligands in Zr-TBB for Type I and Type II PDT to produce four species of reactive oxygen species; (B) time-dependent UV-visible absorption of TBB after light exposure in air-saturated DMF; the percentage of photoproducts (TBB, TBC, fragment) of H4TBB (C) and Zr-TBB (D) after 30 minutes of light irradiation. Reprinted with permission from Luo T, Ni K, Culbert A et al. Nanoscale Metal-Organic Frameworks Stabilize Bacteriochlorins for Type I and Type II Photodynamic Therapy. J Am Chem Soc. 2020;142(16):7334–7339. Copyright (2020) American Chemical Society.Citation54
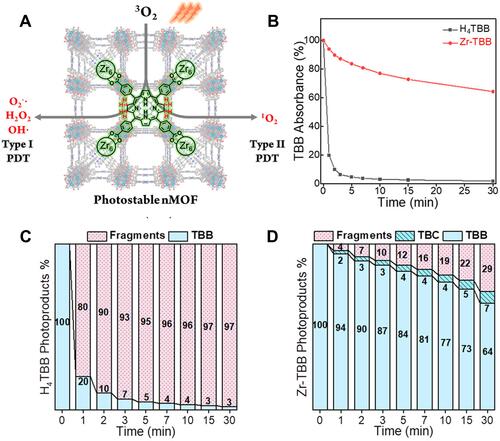
Figure 7 (A) The illustration of preparation process of CuTz-1-O2@F127 and (B) ROS and hypoxia generation in cells after incubation with CuTz-1@F127 or CuTz-1-O2@F127 with or without 808 nm laser irradiation. Scale bar is 50 µm. Reprinted from Cai X, Xie Z, Ding B et al. Monodispersed Copper(I)-Based Nano Metal-Organic Framework as a Biodegradable Drug Carrier with Enhanced Photodynamic Therapy Efficacy. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2019;6(15):1,900,848. © 2019 The Authors. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.Citation100
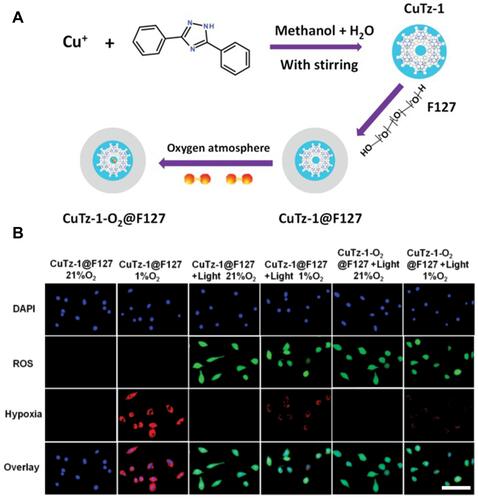
Figure 8 (A) The mechanism of FeTCPP/Fe2O3 exerting antitumor efficacy for enhanced PDT; (B) formation mechanism of the FeTCPP and FeTCPP/Fe2O3 MOF Nanorice. Reprinted with permission from Zhao Y, Wang J, Cai X, Ding P, Lv H, Pei R. Metal-Organic Frameworks with Enhanced Photodynamic Therapy: Synthesis, Erythrocyte Membrane Camouflage, and Aptamer-Targeted Aggregation. ACS Appl Mater Interfaces. 2020;12(21):23,697–23,706. Copyright (2020) American Chemical Society.Citation101
Figure 9 (A) Synthetic scheme to PS@MOF-199 and F127-coated PS@MOF-199 (PS@MOF-199 NPs); (B) quench and trigger of photosensitization originated from PS@MOF-199 NPs in the tumor microenvironment. Reprinted with permission from Wang Y, Wu W, Liu J et al. Cancer-Cell-Activated Photodynamic Therapy Assisted by Cu(II)-Based Metal-Organic Framework. ACS Nano. 2019;13(6):6879–6890. Copyright (2019) American Chemical Society.Citation106
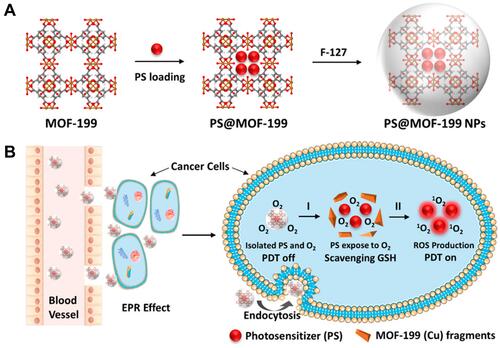
Figure 10 Schematic illustration of all-active MOF nanocarriers for antitumor PDT that involves both apoptosis and ferroptosis. Reprinted with permission from Meng X, Deng J, Liu F et al. Triggered All-Active Metal Organic Framework: Ferroptosis Machinery Contributes to the Apoptotic Photodynamic Antitumor Therapy. Nano Lett. 2019;19(11):7866–7876. Copyright (2019) American Chemical Society.Citation109
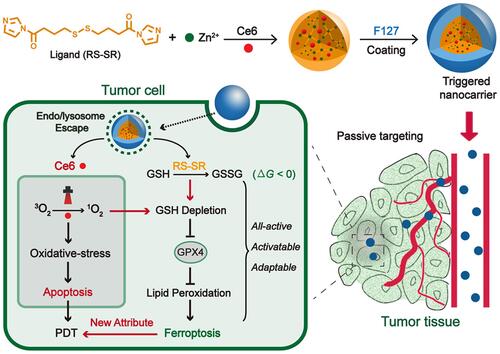
Figure 11 The synthesis process of TPP-SH (A); UiO-66-TPP-SH and TCPP⊂UiO-66 (B); (C) PXRD Figure of UiO-66, TCPP⊂UiO-66, and UiO-66-TPP-SH; (D) SEM and TEM (inset) images of UiO-66-TPP-SH. Scale bar is 200 nm; In vitro cytotoxicity of UiO-66, TPP-SH, TCPP⊂UiO-66, and UiO-66-TPP-SH without (E) and with (F) light irradiation. Reprinted with permission from Kan JL, Jiang Y, Xue A et al. Surface Decorated Porphyrinic Nanoscale Metal-Organic Framework for Photodynamic Therapy. Inorganic chemistry. 2018;57(9):5420–5428. Copyright (2018) American Chemical Society.Citation112
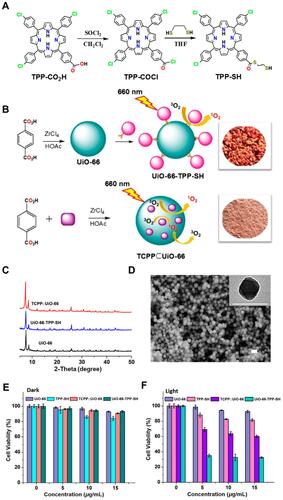
Figure 12 (A) The schematic illustration of the preparation of 1 and artistic representation of its pH-driven selective uptake of cancer and normal cells, and targeting of mitochondria under PDT; (B) perspective view of 2I-BodipyPhNO2; (C) SEM image of 1; (D) PXRD patterns of ZIF-90, 1, and 2I-BodipyPhNO2. The photographs of as-synthesized samples are shown in the inserts. Reprinted with permission from Guan Q, Zhou LL, Li YA, Dong YB. Diiodo-Bodipy-Encapsulated Nanoscale Metal-Organic Framework for pH-Driven Selective and Mitochondria Targeted Photodynamic Therapy. Inorganic chemistry. 2018;57(16):10,137–10,145. Copyright (2018) American Chemical Society.Citation114
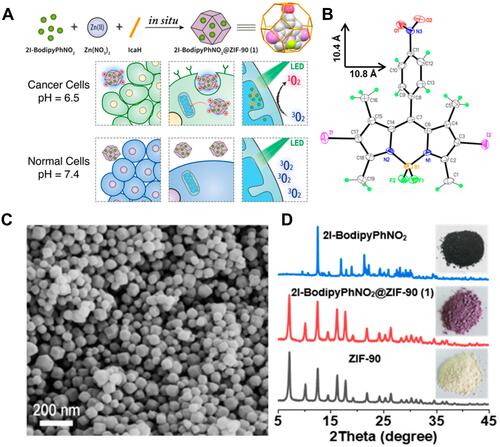
Figure 13 (A) Schematic Illustration for the Formation of a BSA-MnO2/Ce6@ZIF-8 nanoplatform and (B) degradation behavior of BSA-MnO2/Ce6@ZIF-8 NPs dispersed in DI water with pH 5.0; (C) oxygen production ability of Ce6@ZIF-8 and BSA-MnO2/Ce6@ZIF-8 NPs dispersed in DI water with different pH in the presence of H2O2. Reprinted with permission from Sun Q, Bi H, Wang Z et al. O2-Generating Metal–Organic Framework-Based Hydrophobic Photosensitizer Delivery System for Enhanced Photodynamic Therapy. ACS Applied Materials & Interfaces. 2019;11(40):36,347–36,358. Copyright (2019) American Chemical Society.Citation56
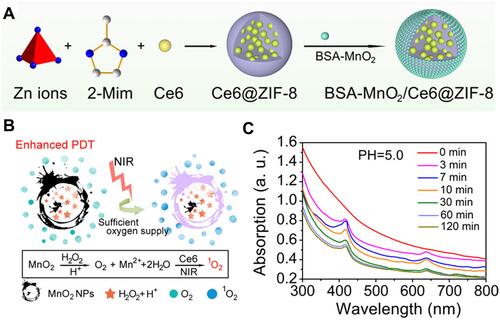
Figure 14 (A) Representation of the preparation process of MCOPP NE; (B) O2 generation of MCOPP NE. The inset images show photographs of H2O2 solutions with and without MCOPP NE; (C) degradation of H2O2 with and without MCOPP NE. Cell viability of 4T1 cells treated in normoxic (D) and hypoxic (E) environments before and after 671 nm laser irradiation; (F) representative immunofluorescence images of tumor slices after hypoxia staining. The hypoxia areas and blood vessels were stained by anti-pimonidazole antibody (green) and anti-CD31 antibody (red); (G) relative hypoxia positive areas and blood vessel density measured by the Image J software; (H) relative tumor volume of mice after treatments (n = 5). Reprinted with permission from Wang D, Wu H, Lim WQ et al. A Mesoporous Nanoenzyme Derived from Metal-Organic Frameworks with Endogenous Oxygen Generation to Alleviate Tumor Hypoxia for Significantly Enhanced Photodynamic Therapy. Adv Mater. 2019;31(27):e1901893. Copyright (2019) WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.Citation57
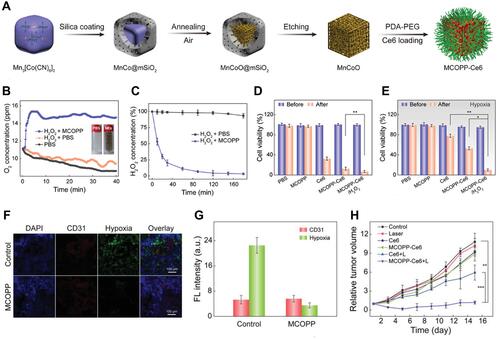
Table 2 The Advantages and Disadvantages of Combination Therapy Compared with PDT
Figure 15 (A) The synthesis process of TPZ/Hf/TCPP/PEG; (B) time-dependent tumor growth curves via tail vein administration at a concentration of 5.0 mg/mL (200 μL); (C) tumor images obtained after different treatments for 14 days. Reprinted with permission from Liu M, Wang L, Zheng X, Liu S, Xie Z. Hypoxia-Triggered Nanoscale Metal-Organic Frameworks for Enhanced Anticancer Activity. ACS Appl Mater Interfaces. 2018;10(29):24,638–24,647. Copyright (2018) American Chemical Society.Citation143
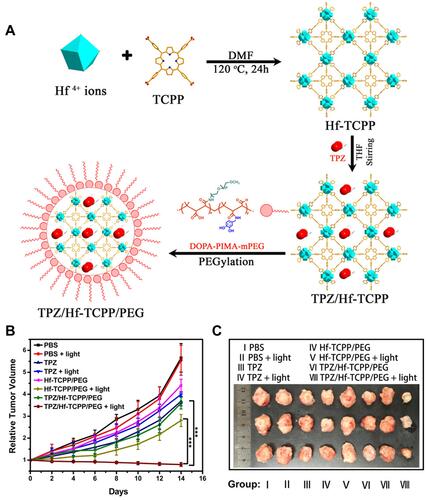
Figure 16 (A) The synthetic process for mCGP; (B) the immune escape and homotypic targeting abilities of mCGP; (C) the cascade reactions amplify the synergistic effects of mCGP for starvation therapy and PDT; (D) schematic illustration of the cascade reactions of mCGP including catalyzing the breakdown of H2O2, decomposing glucose, and producing 1O2; (E) the pH value changes of mCGP solution in the absence and presence of glucose; (F) the O2 concentration changes of mCGP solution in the presence of H2O2 or glucose. Reprinted with permission from Li SY, Cheng H, Xie BR et al. Cancer Cell Membrane Camouflaged Cascade Bioreactor for Cancer Targeted Starvation and Photodynamic Therapy. ACS Nano. 2017;11(7):7006–7018. Copyright (2017) American Chemical Society.Citation49
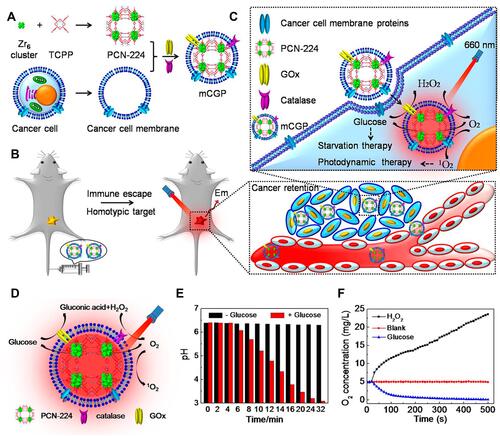
Figure 17 The schematic diagram of synergistic cancer treatment by catalytic cascade enhancement of porphyrin MOF (PCNs)a with double inorganic nanoenzyme engineering. Reprinted with permission from Liu C, Xing J, Akakuru OU et al. Nanozymes-Engineered Metal-Organic Frameworks for Catalytic Cascades-Enhanced Synergistic Cancer Therapy. Nano Lett. 2019;19(8):5674–5682. Copyright (2019) American Chemical Society.Citation80
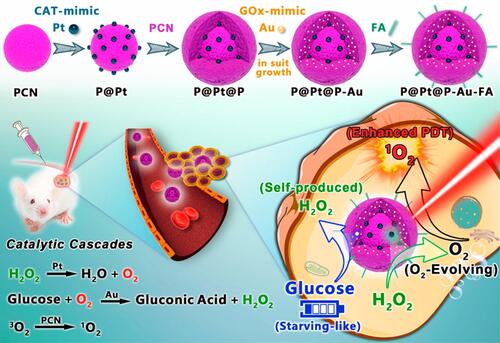
Figure 18 The schematic diagram of TPZ/UCSs and their application to cancer treatment via NIR light-triggered PDT and hypoxia-activated chemotherapy with immunotherapy. Reprinted with permission from Shao Y, Liu B, Di Z et al. Engineering of Upconverted Metal-Organic Frameworks for Near-Infrared Light-Triggered Combinational Photodynamic/Chemo-/Immunotherapy against Hypoxic Tumors. J Am Chem Soc. 2020;142(8):3939–3946. Copyright (2020) American Chemical Society.Citation152
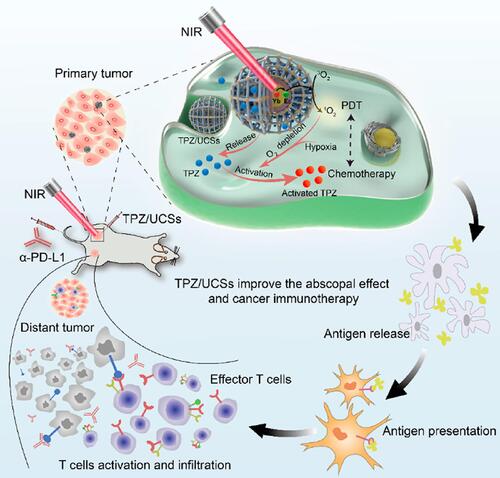
Table 3 A Summary of MOFs for PDT Applications and Their Advantages