Figures & data
Figure 1 The tumor microenvironment components of HCC and their impact on the HCC progression.
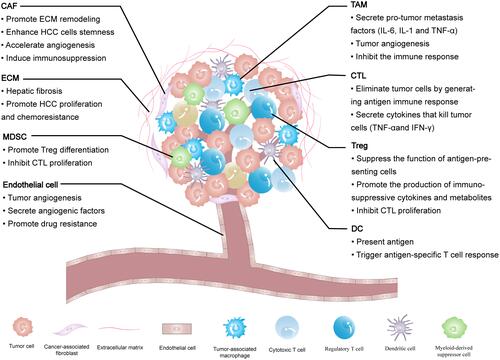
Table 1 The Nanoparticles that Modulate TME and Immune System of HCC
Figure 2 Schematic representation of how Dox-RA-CSNs target liver cancer cells via CD44-mediated endocytosis, and then target the Golgi apparatus via interaction with GalNAc-T.
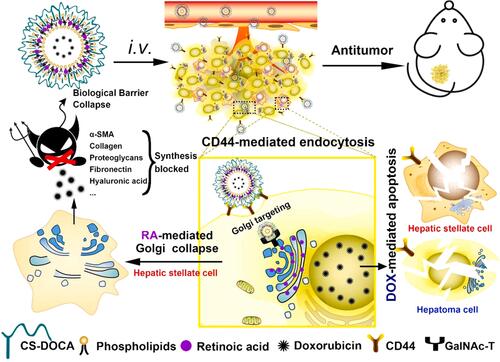
Figure 3 Illustration of PEG-SS-PLL catiomer for siVEGF encapsulation and intracellular stimulus-responsive siVEGF release.
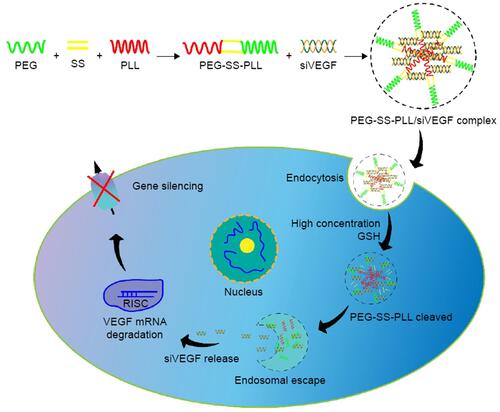
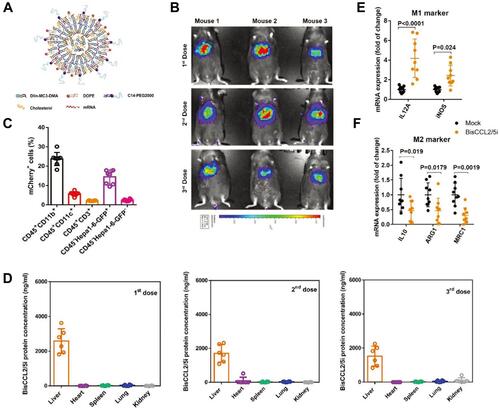
Figure 4 Dual blockade of CCL2 and CCL5 via LNP-mediated mRNA delivery of BisCCL2/5i polarizes the macrophage M1 phenotype and reduces the immunosuppression in the TME. (A) Schematic of the mRNA-loaded LNPs. (B) In vivo transfection of luciferase mRNA-LNPs after repeated administration (i.v., every 4 days, in total 3 doses). The luciferase was injected intraperitoneally into the mice 6 h post the administration of luciferase mRNA-LNPs, followed by measuring the luciferase bioluminescence signal using IVIS imaging. n=3 biologically independent samples. (C) The quantification of mCherry-positive cells expressed in murine orthotopic HCC tumor tissue 6 h after injection of mCherry mRNA-LNPs (mCherry mRNA: 0.5 mg kg−1). mRNA was mainly expressed in monocytes (CD45+CD11b+) and tumor cells (Hepa1-6-GFP+) (n=8 biologically independent mice per group). (D) BisCCL2/5i expression in different organs 6 h after each administration of BisCCL2/5i mRNA-LNPs (mRNA: 1 mg kg−1, i.v., 3 days apart). n=6 biologically independent samples. The BisCCL2/5i mRNA was mainly expressed in the liver tissue and the repeated administration resulted in comparable protein level. (E, F) mRNA expression of classic M1 (E) and M2 (F) markers in the HCC tumor tissues 48 h after systemic administration of formulated LNPs as a dose corresponding to 1 mg kg−1 mRNA (Mock, HcRed mRNA). Each data point is an individual sample (n=9); one-way ANOVA and Tukey’s multiple comparisons test. Change of the immunocellular composition in the HCC TME 48 h following Mock mRNA-LNPs and BisCCL2/5i mRNA-LNPs treatments (mRNA: 1 mg kg−1), measured by flow cytometry (n=4 biologically independent samples; unpaired two-tailed Student’s t-test; the experiment was conducted three times independently with similar results). (G, H) The percentage and cell counts of macrophages (G) and their M2 subtype (H) in the total immune cells. (J, I) Representative flow dots of M1- and M2-phenotype macrophages (J) and the ratio of M1/M2 (I). Data are represented as the mean ± SD.
Notes: Reproduced from Wang Y, Tiruthani K, Li Set al mRNA Delivery of a Bispecific Single-Domain Antibody to Polarize Tumor-Associated Macrophages and Synergize Immunotherapy against Liver Malignancies. Adv Mater. 2021;33(23):e2007603. Copyright © 2021 John Wiley & Sons, Inc. All rights reserved.Citation88
Abbreviations: LNP, lipid nanoparticle; BisCCL2/5i, specific CCL2/CCL5 dual inhibitor; MΦ, macrophages (CD45+CD11b+CD11c−Ly6C−Ly6G−F4/80+).
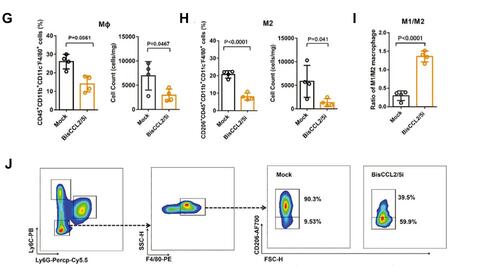
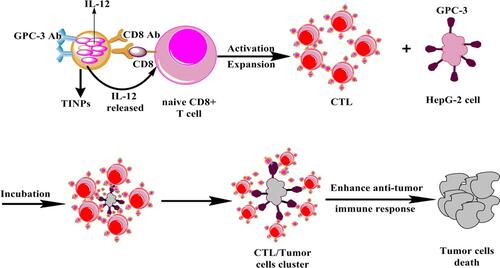
Figure 5 Scheme showing that TINPs enhance CD8+ T cell functions and cancer immunotherapy.