Figures & data
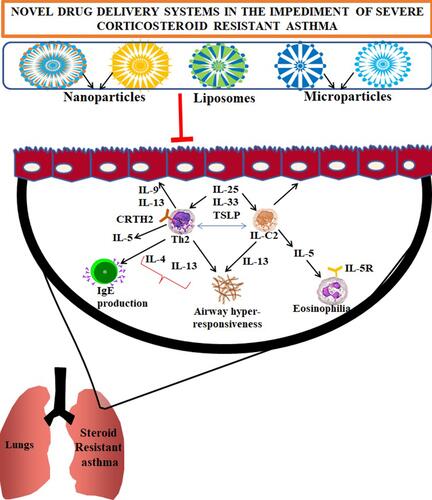
Figure 1 The pathophysiology of asthma and the SSR asthma: Altered genomic mechanisms induced by the repeated intake of glucocorticoids in asthma conditions: Dissociation of glucocorticoid receptor (GR) and the chaperone protein dissociation can induce translocation of GRs into the nucleus. Thus, activation of GRE elements by the translocation of GR activity (when glucocorticoids taken) through specific and nonspecific interactions can provoke the modulation in the activity of HDCS (left panel), and the genome wide changes upon P38MAPK activity (Middle panel). Activation of SSR asthma (right panel) by the intake of allergens or due to infections through the formation of NLRP inflammasome; the administration of MCC950, YVA, and α-IL-1β to impair the pathophysiology induced through NLRP inflammasome and release of intracellular content that cause airway inflammation.
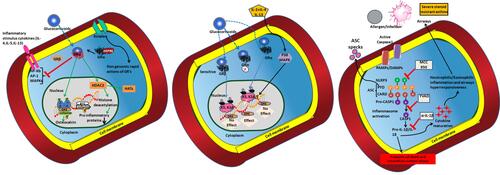
Figure 2 Mitigation in the SSR asthma by modulating the miR-21/PTEN/PI3K/HDAC2 signaling suggesting the significant role of miR-21. Ant-21 and a pan-PI3K inhibitor LY294002 treatment mitigated the activity of PI3K and retrieved HDAC2 levels subsequently impaired the airway hyperresponsiveness and enhanced the steroid sensitivity to allergic airway disease.
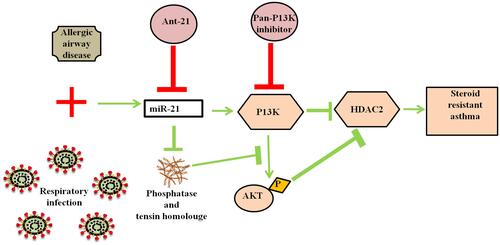
Figure 3 The pathophysiology and various antiasthmatic therapeutic modalities in the form of nanoformulations in treating asthma: Anti-IL-4Ra (ex. dupilumab), Anti-IgE (omalizumab), CRTH2 antagonist (Fevipiprant), antiIL-5R (benralizumab), Anti-IL-5 (meprolizumab), anti-TSLP (tezepelumab), chemokine receptors blockers, CCR5 modulators, specific kinase blockers, corticosteroids, chemokine receptor blockers, antiallergy blockers, cytokine agonists are prominent therapeutic strategies to target asthma pathophysiology; the nanoformulations (liposomes, nanoparticles, microparticles) by inducing surface modifications can enable responsive drug release against disease-specific enzymes. The therapeutic formulation loaded with antiasthmatic drugs can minimize the underlying pathophysiology of asthma or SSR (severe steroid resistant) asthma.
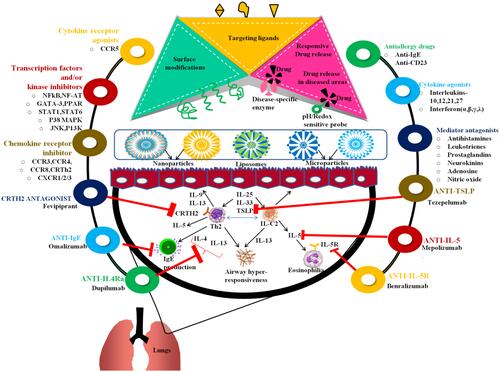
Table 1 Various Nanocarrier Compositions (Liposome-Based Formulations) and the Method of Preparations and Their Mode of Actions in Targeting AADs Including Allergic Asthma: Possible Future Implications Against SSR Asthma
Table 2 Various Nanocarrier Compositions (Nanoparticles) Formulated for Several Therapeutic Molecules and the Method of Preparations and Their Mode of Actions in Targeting AADs Including Allergic Asthma: Possible Future Implications Against SSR Asthma
