Figures & data
Figure 1 Glucose metabolism adaptation in cancer cells and drugs targeting glucose metabolism processes that included in the review. Malignant cells exhibit high anabolic rates. They prefer consuming large amounts of glucose for energy supplement and synthesis of biological essentials, including nucleotides, amino acids, and lipids. Agents disrupting glucose metabolism are predicted to result in a lack of energy and resources required for cell proliferation, leading to the death of neoplasm cells.
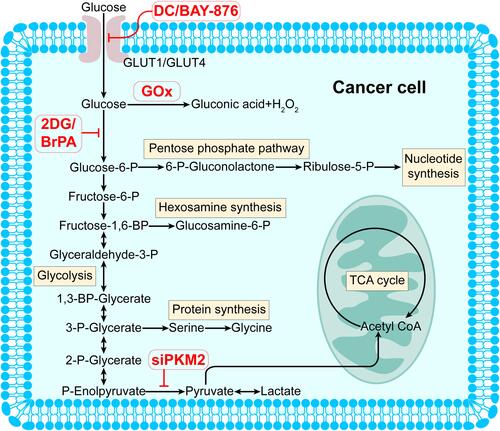
Figure 2 Schematic illustration of nanomaterial-assisted cancer starvation therapy targeting glucose metabolism.
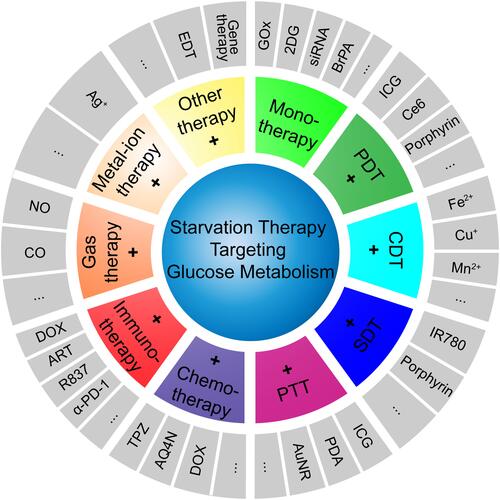
Table 1 Summary of Nanodrugs Targeting Glucose Metabolism
Figure 3 Schematic illustration for the mechanism of autophagy inhibition-augmented tumor-ST. Step 1: 2DG is applied to restrain glycolysis and initiate severe starvation of cancer cells. Step 2: BP nanosheet inhibits downstream protective autophagy, cuts off the compensatory nutrition supply and finally promotes apoptosis. Reproduced with permission from: Yang B, Ding L, Chen Y, Shi J. Augmenting Tumor-Starvation Therapy by Cancer Cell Autophagy Inhibition. Adv Sci (Weinh). 2020;7(6):1902847. doi:10.1002/advs.201902847.Citation38 Copyright 2020 The Authors. Creative Commons Attribution License.
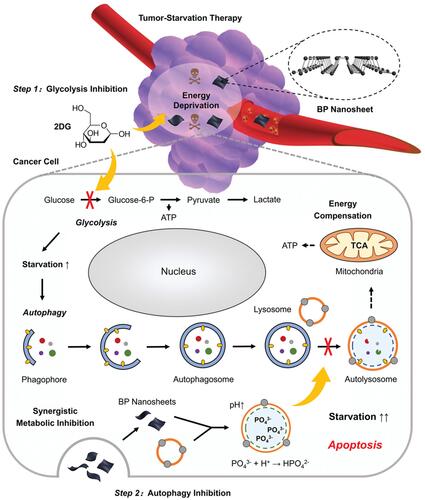
Figure 4 Schematic illustration of engineered liposome nanomedicines and their interaction with cancer cells and normal cells enabling differential stress sensitization of chemotherapy. Reproduced with permission from: Yang B, Chen Y, Shi J. Tumor-Specific Chemotherapy by Nanomedicine-Enabled Differential Stress Sensitization. Angew Chem Int Ed Engl. 2020;59(24):9693–9701. doi:10.1002/anie.202002306.Citation65 Copyright 2020, Wiley-VCH.
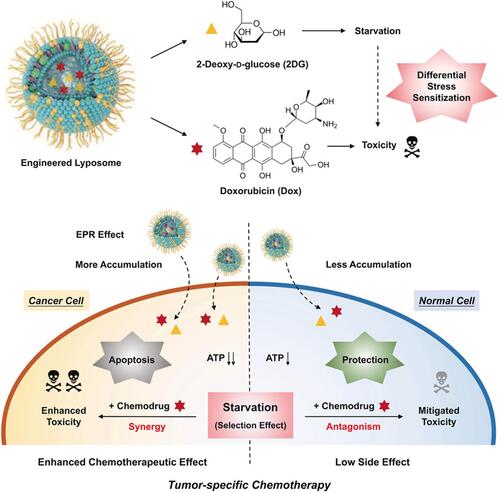
Figure 5 (A) Schematic illustration of the structure of P-B-D NPs. (B) Schematic illustration of the anti-tumor mechanism for synergistic ST/chemotherapy. Reproduced with permission from: Jiang W, Luo X, Wei L, et al. The Sustainability of Energy Conversion Inhibition for Tumor Ferroptosis Therapy and Chemotherapy. Small. 2021;17(38):2102695. doi:10.1002/smll.202102695.Citation30 Copyright 2021, Wiley-VCH.
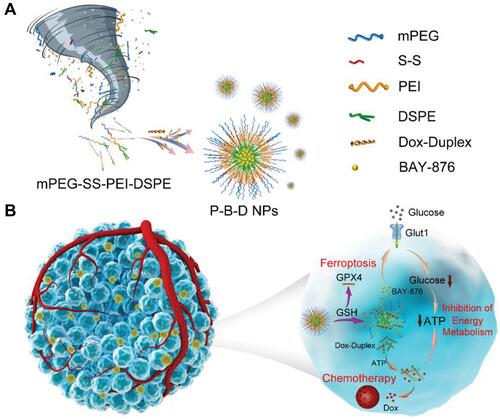
Figure 6 Synthetic route of UCCG and TME-activated enzymatic cascade catalytic reaction for synergistic cancer ST/CDT/immunotherapy process. Reproduced with permission from: Wang M, Chang M, Li C, et al. Tumor-Microenvironment-Activated Reactive Oxygen Species Amplifier for Enzymatic Cascade Cancer Starvation/Chemodynamic /Immunotherapy. Adv Mater. 2021;34(4):e2106010. doi:10.1002/adma.202106010.Citation86 Copyright 2021, Wiley-VCH.
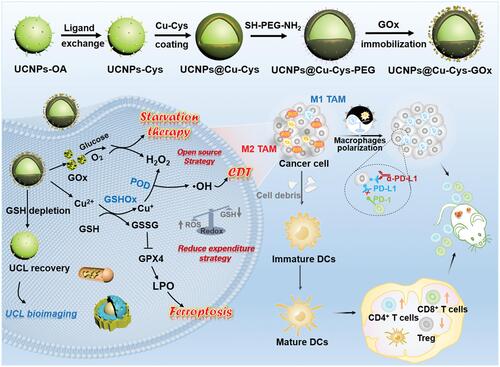
Figure 7 Schematic diagram of PEGylated Au@HMnMSNs ICD nanoinducers for eliciting potent antitumor immunotherapeutic efficacy. Reproduced with permission from: Sun K, Hu J, Meng X, et al. Reinforcing the Induction of Immunogenic Cell Death Via Artificial Engineered Cascade Bioreactor-Enhanced Chemo-Immunotherapy for Optimizing Cancer Immunotherapy. Small. 2021;17(37):e2101897. doi:10.1002/smll.202101897.Citation90 Copyright 2021, Wiley-VCH.
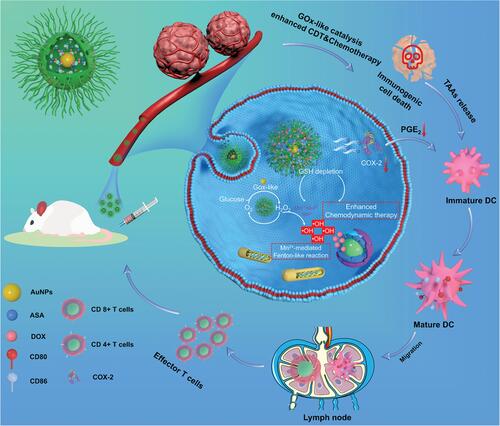
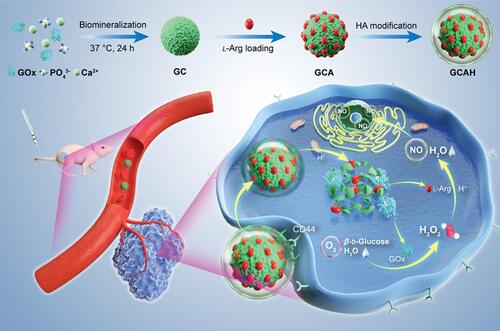
Figure 8 Schematic illustration of the preparation process of GCAH and its application for synergistic cancer ST/gas therapy. Reproduced with permission from: Fu LH, Li C, Yin W, et al. A Versatile Calcium Phosphate Nanogenerator for Tumor Microenvironment-activated Cancer Synergistic Therapy. Adv Healthc Mater. 2021;10(23):e2101563. doi:10.1002/adhm.202101563.Citation198 Copyright 2021, Wiley-VCH.