Figures & data
Table 1 Nanoparticles synthesized from different plants and their size range
Figure 1 Optical photograph of colloidal solution of (A) Trianthema decandra root extract, (B) HAuCl4 solution reduced with 5 mL of T. decandra extract, (C) HAuCl4 solution reduced with 10 mL of T. decandra extract, and (D) HAuCl4 solution reduced with 15 mL of T. decandra extract.
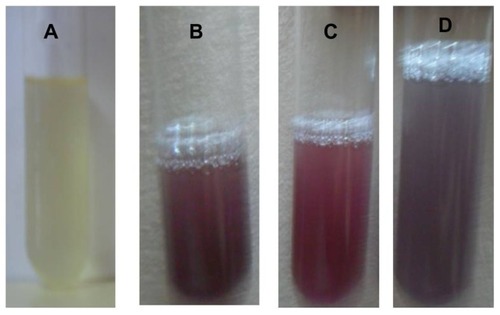
Figure 2 Optical photograph of colloidal solution of (A) Trianthema decandra root extract, (B) AgNO3 solution reduced with 5 mL of T. decandra extract, (C) AgNO3 solution reduced with 10 mL of T. decandra extract, and (D) AgNO3 solution reduced with 15 mL of T. decandra extract.
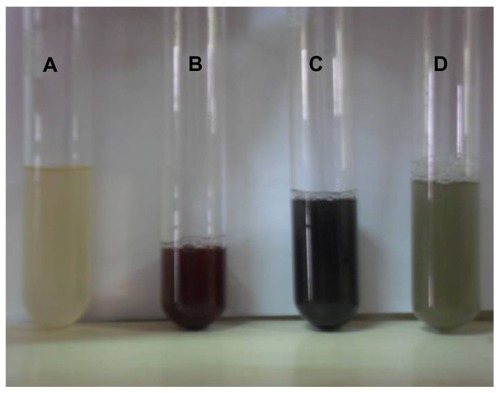
Figure 3 Ultraviolet-visible bioreduction kinetics in the 20–800 nm range for colloidal HAuCl4 solution with 5 mL of Trianthema decandra root extract (A), 10 mL of T. decandra root extract (B), and 15 mL of T. decandra root extract (C).
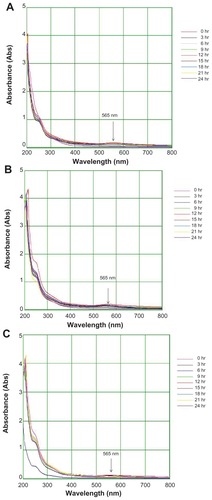
Figure 4 Ultraviolet-visible bioreduction kinetics in the 200–800 nm range for colloidal AgNO3 solution with 5 mL of Trianthema decandra root extract (A), 10 mL of T. decandra root extract (B), and 15 mL of T. decandra root extract (C).
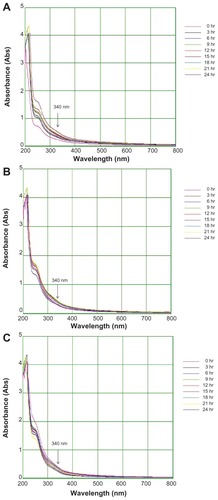
Figure 5 Fourier transform infrared absorption spectra for nanoparticles synthesized by bioreduction of HAuCl4 ions using Trianthema decandra root extract.
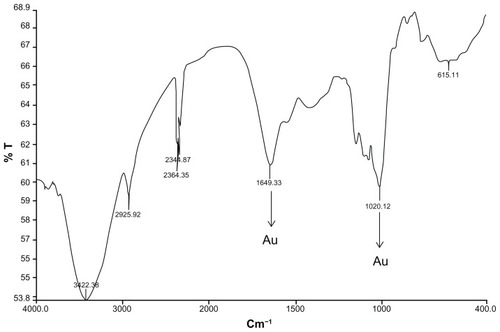
Figure 6 Fourier transform infrared absorption spectra for nanoparticles synthesized by bioreduction of AgNO3 ions using Trianthema decandra root extract.
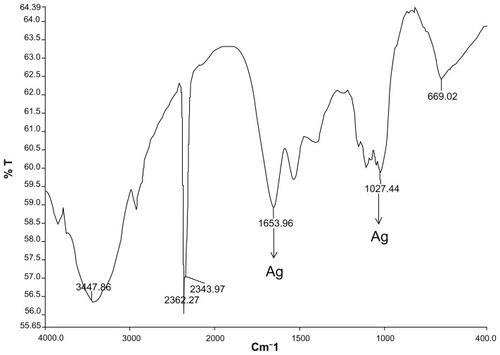
Figure 7 Scanning electron micrographs of gold nanoparticles observed from the reaction of Au3+ cations with Trianthema decandra root extract.
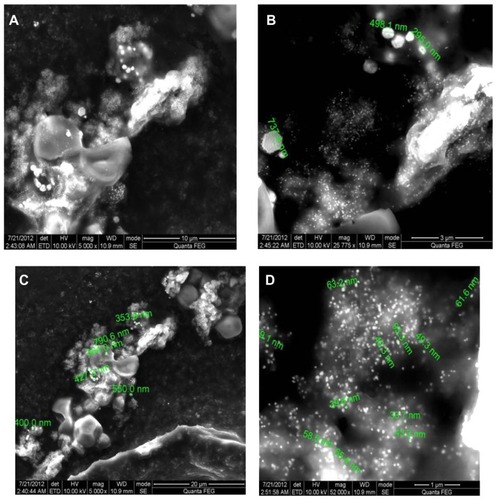
Figure 8 Scanning electron micrographs of silver nanoparticles observed from reaction of Ag+ cations with Trianthema decandra root extract.
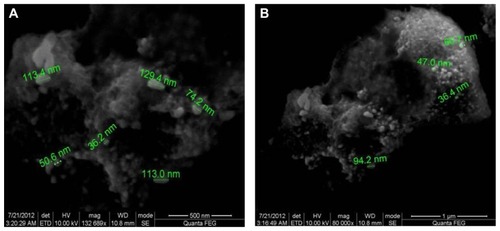
Figure 9 Energy dispersive x-ray spectroscopy of gold nanoparticles synthesized by Trianthema decandra root extract.
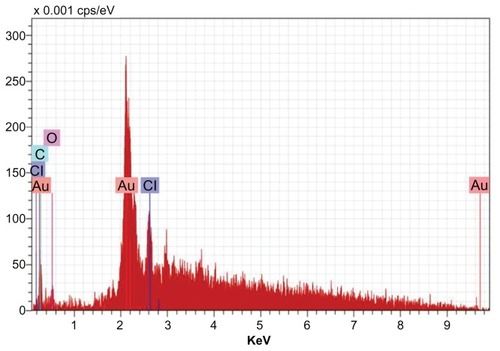
Figure 10 Energy dispersive x-ray spectroscopy of silver nanoparticles synthesized by Trianthema decandra root extract.
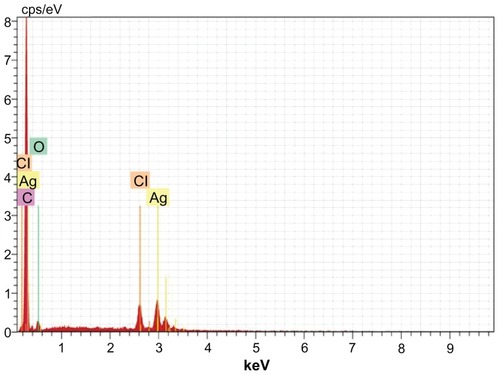
Table 2 Mean zone of inhibition of synthesized gold and silver nanoparticles from T. decandra
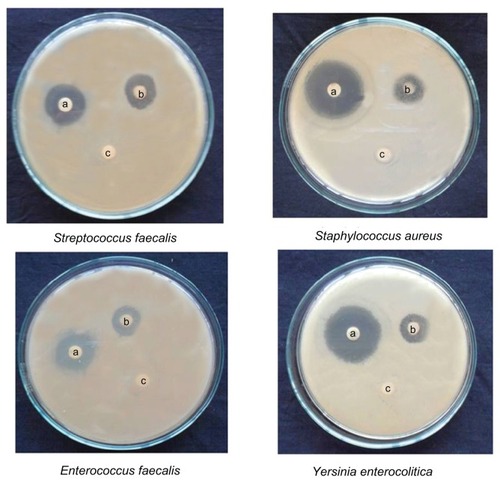
Figure 11 Activity of gold nanoparticles against different microorganisms depicting zones of inhibition of (A) positive control. (B) gold nanoparticles, and (C) dimethylsulfoxide control.
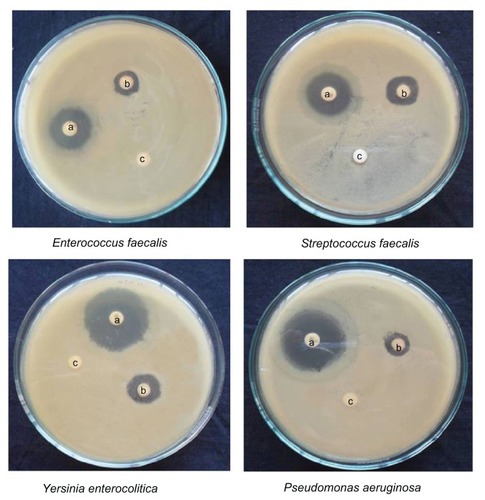
Figure 12 Activity of silver nanoparticles against different micro-organisms depicting zones of inhibition of (A) positive control. (B) silver nanoparticles, and (C) dimethylsulfoxide control.