Figures & data
Figure 1 The characteristics and healing mechanisms of biomembrane-based nanostructure- and microstructure-loaded hydrogels for chronic wounds. Nanostructures and microstructures can be loaded into hydrogels by various methods to produce carriers that have specific properties that can promote wound healing through different mechanisms.
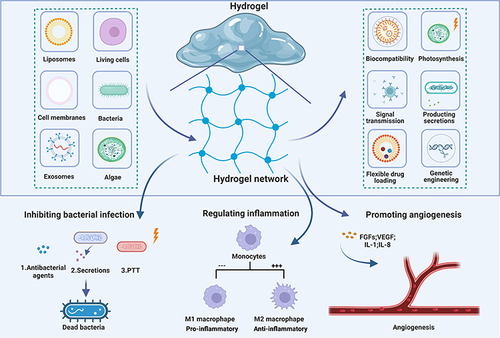
Table 1 Representative Examples of Biomembrane-Based Nano- and Microstructure-Loaded Hydrogels in Diabetic Wounds
Figure 2 Anatomical and molecular mechanisms of Th and Th-Lipo-loaded GelMA hydrogel repairing and regenerating diabetic skin. Reprinted from Chemical Engineering Journal, 397, Wang C, Wu T, Liu G, et al. Promoting coagulation and activating SMAD3 phosphorylation in wound healing via a dual-release thrombin-hydrogel, 125414, Copyright © 2020, with permission from Elsevier B.V.Citation101
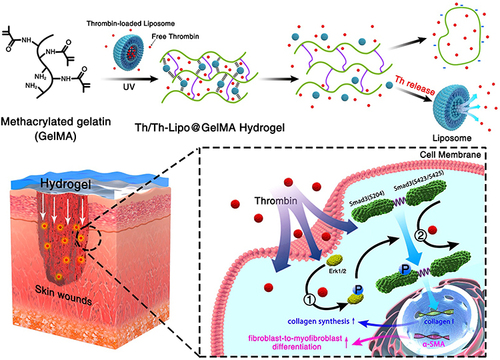
Figure 3 Diagram illustrating how the RBCM scaffold was fabricated. Reprinted from Biomaterials, 197, Fan Z, Deng J, Li PY, et al. A new class of biological materials: Cell membrane-derived hydrogel scaffolds, 244-254, Copyright © 2019, with permission from Elsevier.Citation126
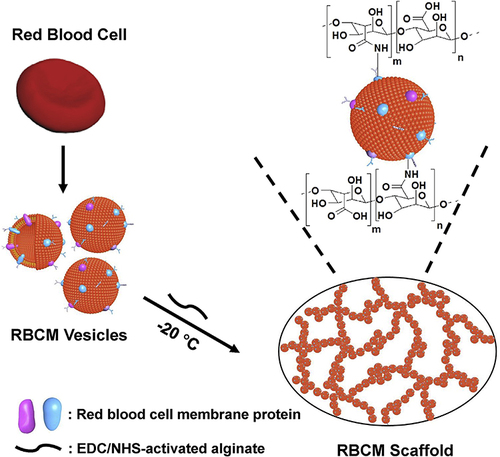
Figure 4 (A) Schematic diagram of the self-healing process of hydrogels. (B) pH-dependent release of exosomes from hydrogels. (C) The healing process of wounds treated with hydrogel, exosomes, exosome-loaded hydrogel and control wounds. Adapted with permission from Wang C, Wang M, Xu T, et al. Engineering Bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9:65–76, Open Access.Citation133 (D) Hydrogel consisting of Cothe HA@MnO2/FGF-2/Exos is formed by the Schiff base reaction of hydrazides and aldehydes. (E) HA@MnO2/FGF-2/Exos hydrogel can be quickly formed by simple mixing injections, in which grafted ortho-quaternary ammonium groups have long-term antibacterial efficacy. The MnO2/ε-PL nanosheet is a nanoenzyme that catalyzes the oxidation of H2O2 into O2. The release of ExosM2@miR−223 mimicked persistent angiogenesis, and the release of FGF-2 enhanced epithelial formation. Reprinted with permission from Xiong Y, Chen L, Liu P, et al. All-in-One: multifunctional Hydrogel Accelerates Oxidative Diabetic Wound Healing through Timed-Release of Exosome and Fibroblast Growth Factor. Small. 2022;18:e2104229.© 2021 The Authors. Small published by Wiley-VCH GmbH.Citation142
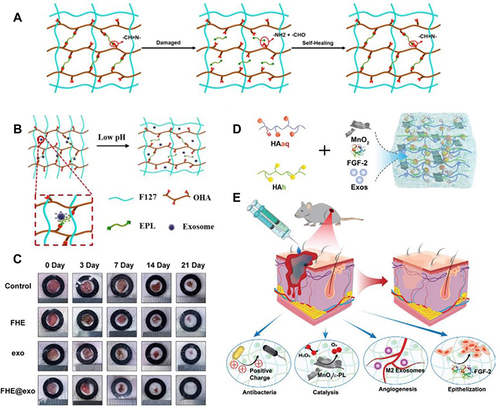
Figure 5 Diagram of MSC-loaded hydrogels for chronic wounds. Adapted with permission from Chen S, Shi J, Zhang M, et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep. 2015;5:18104, Open Access.Citation162
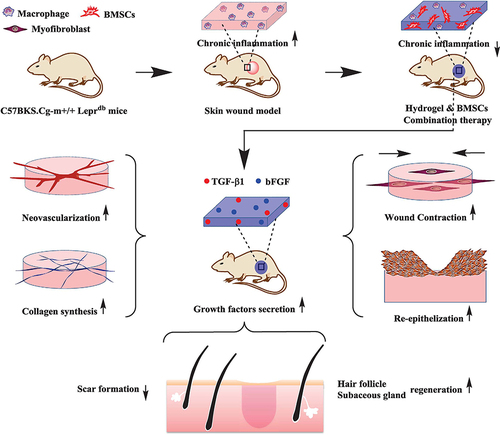
Figure 6 Schematic illustration of the preparation and acceleration of wound healing while using living probiotic hydrogels. (A) The process of encapsulating bacteria in microspheres. (B) Covalent cross-linking within light-irradiated microspheres containing active probiotics. (C) Procedure for treating wounds with live bacterial hydrogel. Reprinted from Ming Z, Han L, Bao M, et al. Living bacterial hydrogels for accelerated infected wound healing. Adv Sci. 2021;8:e2102545, © 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.Citation171
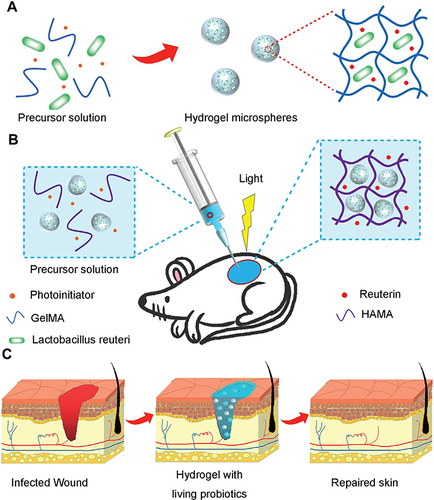
Figure 7 (A) Schematic for fabricating PSB gel dressings to eliminate MRSA and accelerate wound healing. (B) Curves of photothermal temperature for PBS, PSB, and inactivated PSB irradiated with an 808 nm (1 W/cm2) laser. (C) Images of PSB, inactivated PSB, and phosphate-buffered saline exposed to an 808 nm laser (1 W/cm2) infrared thermography. (D) Antibacterial properties of PBS in vitro. (E) H&E staining of wound tissue. The data were presented as the mean ± standard deviation (SD) of measurements (*p < 0.05, **p < 0.01, and ***p< 0.001). Adapted from Acta Biomater, 140, Zhao E, Liu H, Jia Y, et al. Engineering a photosynthetic bacteria-incorporated hydrogel for infected wound healing. 302–313, Copyright 2022, with permission from Elsevier.Citation174
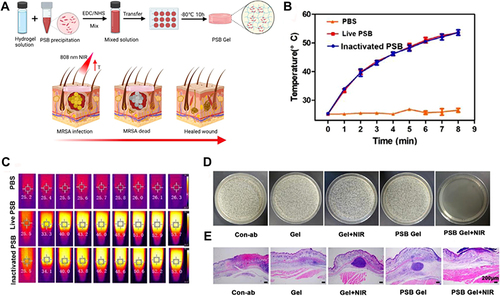
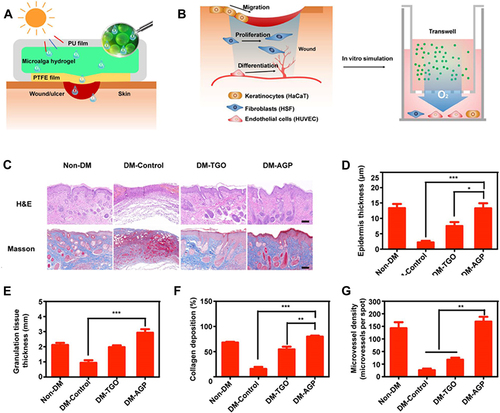
Figure 8 (A) Diagram illustrating the preparation of AGP and the treatment of diabetic wounds by dissolved oxygen release in response to light. (B) Design scheme of the wound-healing process using algae gel. (C) Different groups of wounds showed regenerated skin by H&E and Masson staining at day 12. (D-G) Quantification of the epithelial gap (D), granulation tissue (E), collagen deposition (F) and average microvessel densities in different groups (G). Significantly different (one-way ANOVA): *P<0.05, **P<0.01, and ***P<0.001. Adapted from Chen H, Cheng Y, Tian J, et al. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci Adv. 2020;6:eaba4311, Open Access.Citation62